Organic Compounds Containing Oxygen
Organic Compounds Containing Oxygen PDF Notes, Important Questions and Synopsis
SYNOPSIS
1. Alcohols (R-OH) and Phenols (Ph-OH):
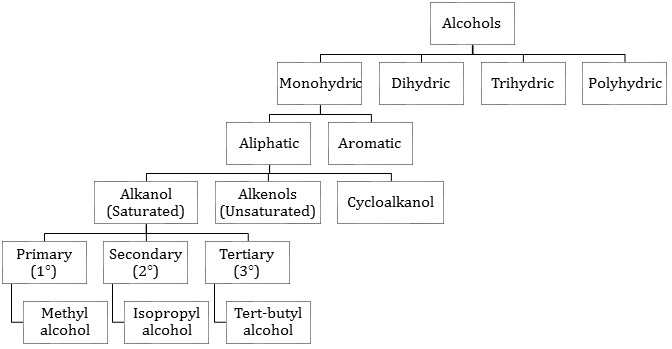
- Classification of alcohols:
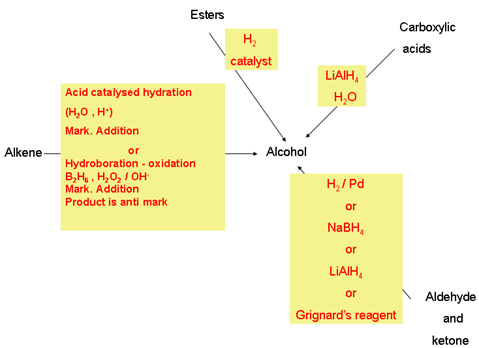
- Preparation Alcohols:
- Preparation of phenols
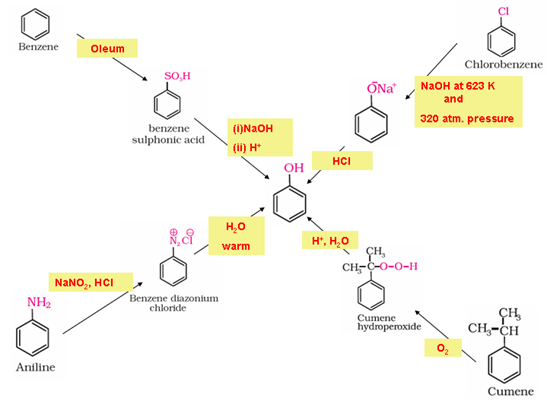
- Chemical properties:
Alcohols react both as nucleophiles and electrophiles.
- Reactions involving cleavage of O-H bond
i. Reaction with metals
Alcohols and phenols react with active metals like Na, K and Al to give corresponding alkoxides/phenoxides with the evolution of hydrogen.
2R-OH + 2Na → 2R-O-Na + H2
ii. Esterification
i. Esters are formed when alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides.
Esters are formed when alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides.
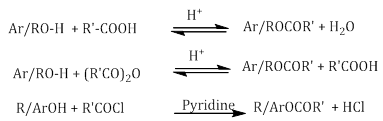
ii. The reaction takes place in the presence of small amount of conc. sulphuric acid in case of carboxylic acid and acid anhydride. The reaction is reversible and therefore water is removed as soon as it is formed.
- Reactions involving cleavage of Carbon-Oxygen(C-O) bond in alcohols
Only alcohols show reactions involving cleavage of C-O bond. Phenols exhibit this type of reaction only with zinc.
i. Reaction with hydrogen halides
Alcohols on treatment with hydrogen halides form alkyl halides.
ROH + HX → R-X + H2O
ii.Reaction with Phosphorus trihalides
Alcohols get converted into alkyl bromides on treatment with PBr3.
3R-OH + PBr3 → 3R-Br + H3PO3
iii.Dehydration
- Alcohols undergo dehydration to form alkenes in the presence of conc. H2SO4 or H3PO3 or catalysts such as anhydrous zinc chloride or alumina.
- Primary alcohol undergoes dehydration by heating it with conc. H2SO4 at 443K
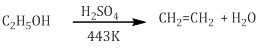
- Secondary and tertiary alcohols undergo dehydration in milder conditions.
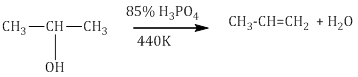
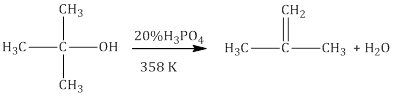
- Thus the ease of dehydration of alcohols follows the order:
Tertiary > Secondary > Primary
iv.Oxidation
The oxidation of alcohols results in the formation of a carbon-oxygen double bond with the cleavage of an O-H and C-H bonds. The reaction is known as dehydrogenation reaction as it involves loss of dihydrogen from an alcohol molecule. 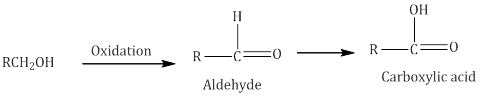
Use of strong oxidising agents like acidified KMnO4 is done to obtain carboxylic acids from alcohols directly. CrO3 in anhydrous medium is used for obtaining aldehydes. CrO3 is used to oxidize secondary alcohols to ketones.
Pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl is a better oxidizing agent for oxidation of primary alcohols to aldehydes in good yield.
- Chemical properties of phenols
- Phenols show electrophilic substitution reactions.
- The –OH group activates the benzene ring towards electrophilic substitution and also directs the incoming group to ortho and para positions in the ring as these positions become electron rich due to the resonance effect caused by –OH group.
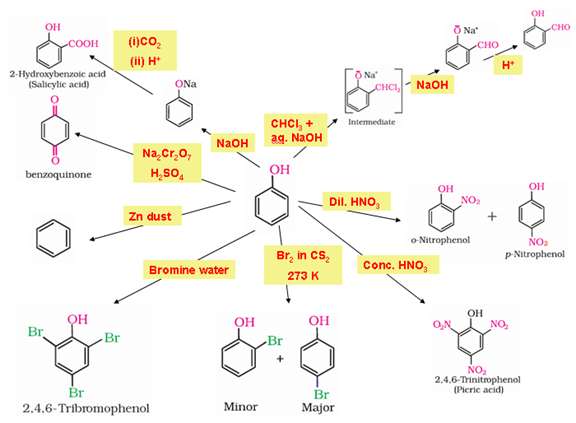
- Acidic nature
- Phenol > H2O > Primary alcohol > Secondary alcohol > Tertiary alcohol
The acidic character of alcohols is due to the polar nature of the O–H bond.
- How to distinguish between some important pair of organic compounds
- Phenol and alcohol:
Phenol on reaction with neutral FeCl3 gives purple colour, whereas alcohols do not give purple colour.
6C6H5OH + Fe3+ → [Fe(OC6H5)6]3− + 6H+
Purple colour - Primary, secondary and tertiary alcohol:
Lucas reagent test: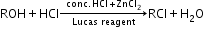
If it is a primary alcohol, then no turbidity appears at room temperature. Turbidity appears only on heating.
If it is a secondary alcohol, then turbidity appears in 5 minutes.
If it is a tertiary alcohol, then turbidity appears immediately. - Methanol and ethanol:
Iodoform test:
Ethanol when reacted with I2 and NaOH or NaOI gives a yellow ppt. of iodoform because of the presence of the CH3–CH (OH)− group.
C2H5OH + 4I2+ 6NaOH → CHI3 + 5NaI + 5H2O + HCOONa
Yellow ppt.
CH3OH + I2+ NaOH → No yellow ppt.
2. Ethers (R-O-R’):
- Preparation:
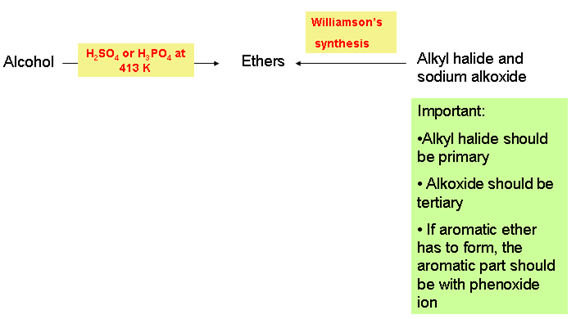
- Chemical properties:
- Cleavage of C–O bond in ethers
- Since ethers are least reactive of the functional groups, the cleavage of C-O bond in ethers takes place in excess of hydrogen halides.

- The cleavage of ethers with two different alkyl groups also takes place in the same manner.

The order of reactivity of hydrogen halides is:
HI > HBr > HCl
- Since ethers are least reactive of the functional groups, the cleavage of C-O bond in ethers takes place in excess of hydrogen halides.
- Electrophilic substitution reaction in aromatic ethers:
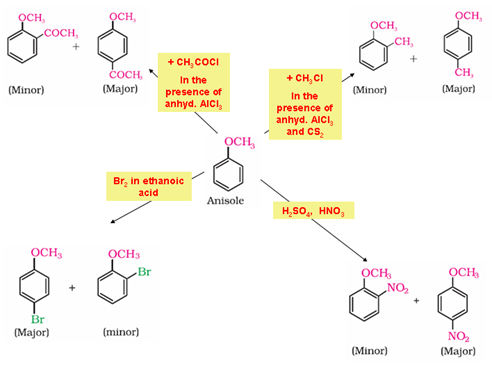
3. Reactions of Aldehydes and Ketones
Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons (or inductive effect).
- Nucleophilic addition reactions of aldehydes and ketones
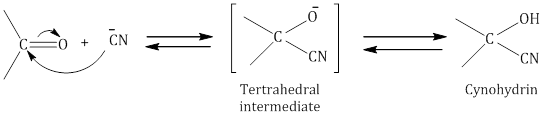
- Addition of hydrogen cyanide (HCN) to form cyanohydrins:
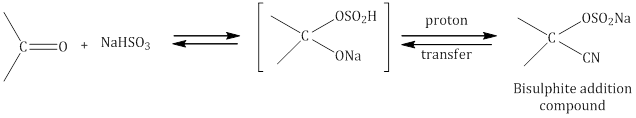
- Addition of sodium hydrogen sulphite (NaHSO3) to form bisulphate addition compound:
- Addition of Grignard reagent (RMgX) to form alcohol:
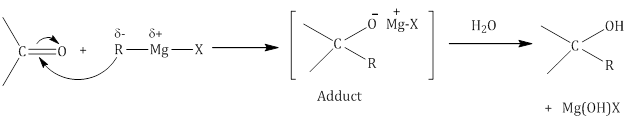
- Addition of alcohol:
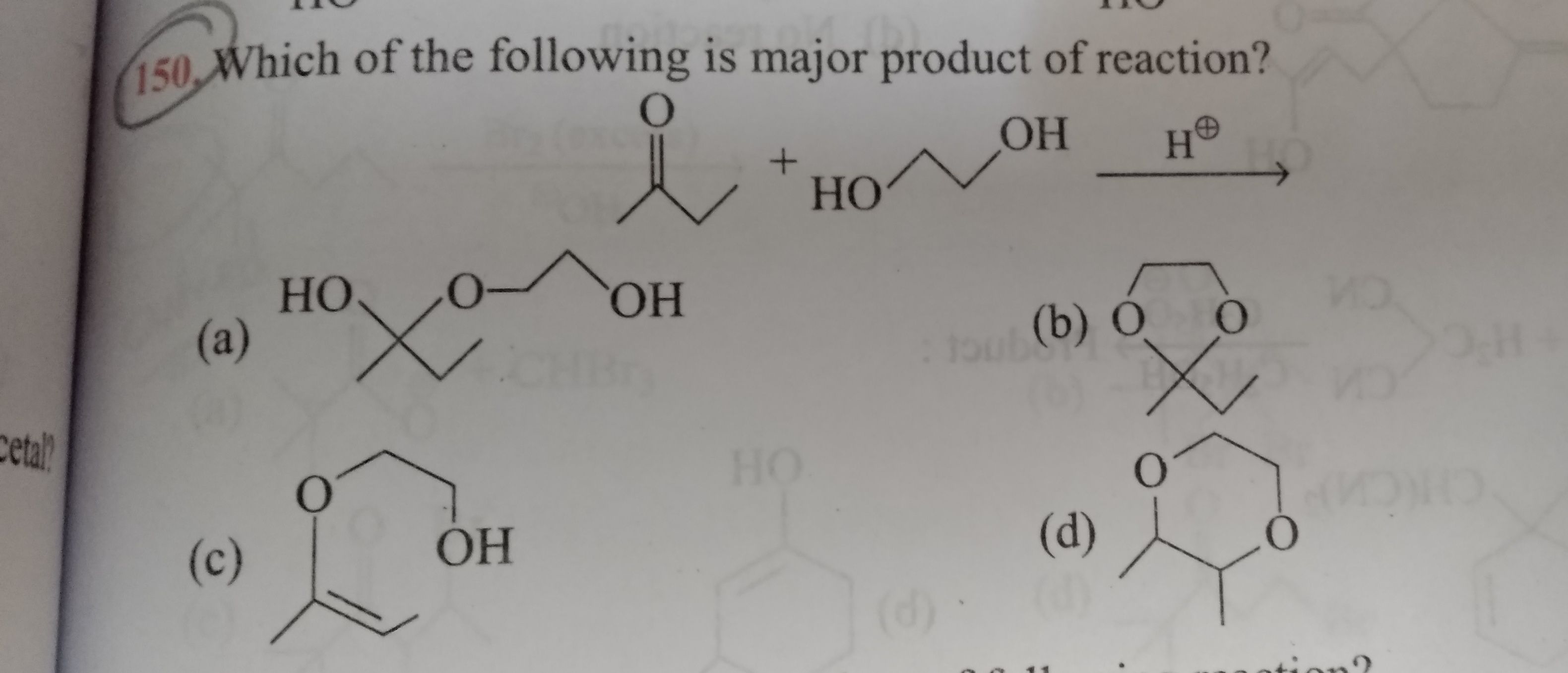
i. On addition of monohydric alcohol in the presence of dry HCl, aldehydes form hemiacetal and acetal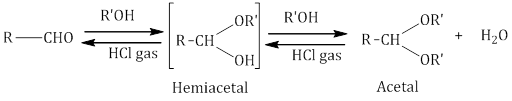 Ketones do not ii. react with monohydric alcohols. Ketones react with ethylene glycol under similar conditions to form cyclic products known as ethylene glycol ketals.
Ketones do not ii. react with monohydric alcohols. Ketones react with ethylene glycol under similar conditions to form cyclic products known as ethylene glycol ketals.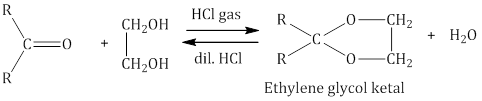
- Addition of ammonia and its derivatives:
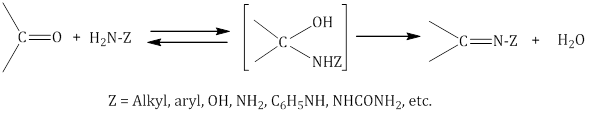
- Addition of hydrogen cyanide (HCN) to form cyanohydrins:
- Reduction of aldehydes and ketones
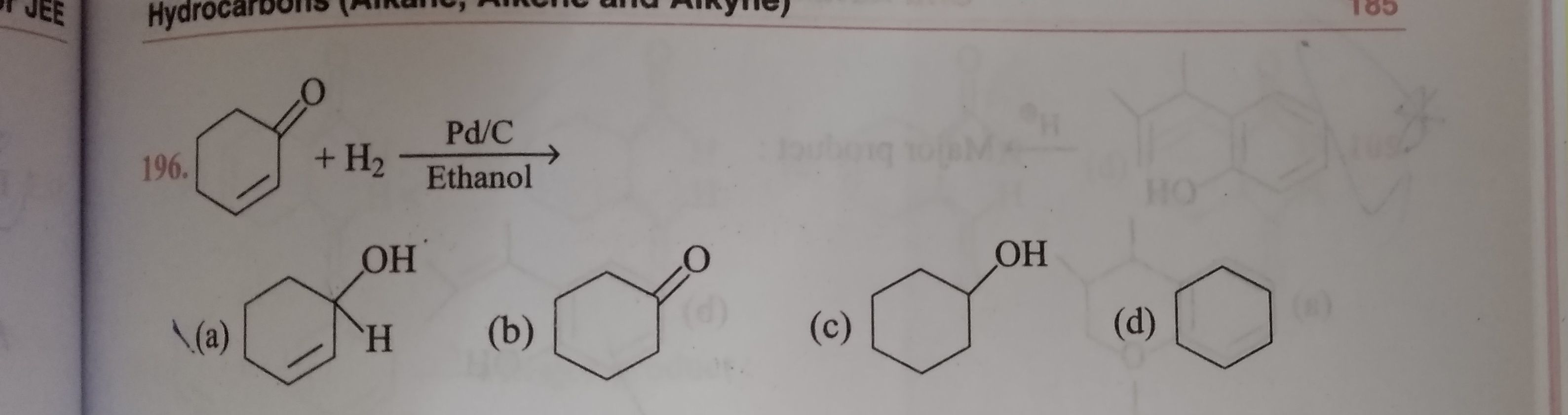
a. Reduction to alcohols: On catalytic hydrogenation in the presence of Ni, Pt or Pd by using lithium aluminium hydride ( ) or sodium borohydride ( ), aldehydes and ketones form primary and secondary alcohols, respectively.
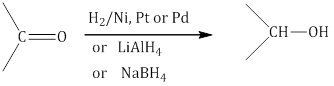
b.Reduction to hydrocarbons:
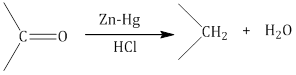
i. Clemmensen reduction: The carbonyl group of aldehydes and ketones is reduced to the CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid.
ii. Wolff–Kishner reduction: The carbonyl group of aldehydes and ketones is reduced to the CH2 group on treatment with hydrazine followed by heating with sodium or potassium hydroxide in a high boiling solvent such as ethylene glycol.
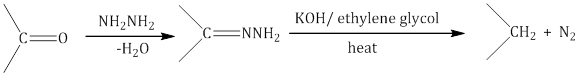
- Oxidation of aldehydes and ketones:
- Aldehydes are oxidised to acids in the presence of mild oxidising agents HNO3, K2Cr2O7, KMnO4.
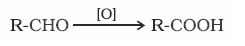
- Ketones are generally oxidised under drastic conditions, i.e. with powerful oxidising agents such as conc. HNO3, KMnO4/H2SO4 and K2Cr2O7/H2SO4 at elevated temperature.
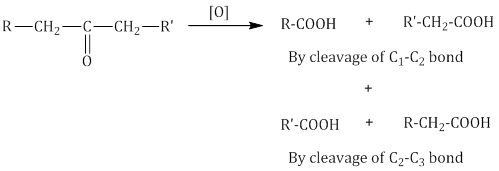
Important Note: In unsymmetrical ketones, cleavage occurs in such a way that the keto group remains with the smaller alkyl group. This is known as the Popoff’s rule. - Haloform reaction: Aldehydes and ketones with at least one methyl group linked to the carbonyl carbon atom, i.e. methyl ketones are oxidised by sodium hypohalite to sodium salts of corresponding carboxylic acids with one carbon atom less than that of the carbonyl compound. The methyl group is converted to haloform.
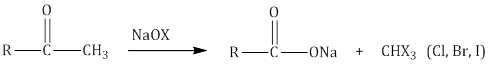
Reactions of aldehydes and ketones due to α-hydrogen:
- Aldol condensation: Aldehydes and ketones with at least one α-hydrogen undergo self-condensation in the presence of dilute alkali as a catalyst to form α-hydroxy aldehydes (aldol) or α-hydroxy ketones (ketol), respectively.
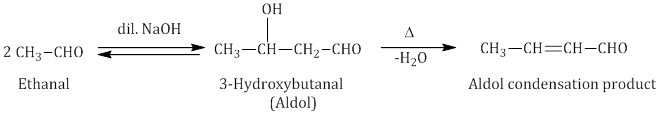
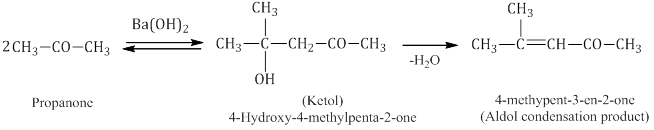
- Cross aldol condensation: Aldol condensation between two different aldehydes and ketones is called aldol condensation. If both of them contain α-hydrogen atoms, it gives a mixture of four products.
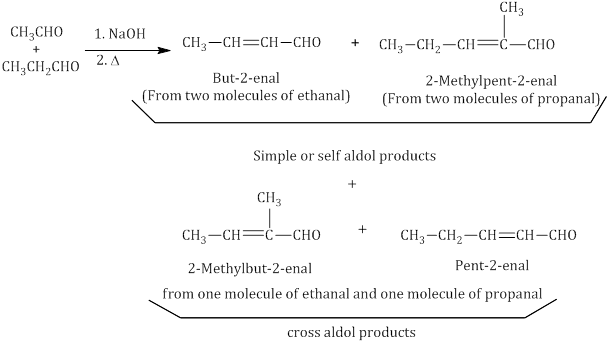
Cannizzaro reaction:
Aldehydes which do not have an α-hydrogen atom undergo self-oxidation and reduction (disproportionation) reaction on treatment with concentrated alkali to form alcohol and salt of acid.
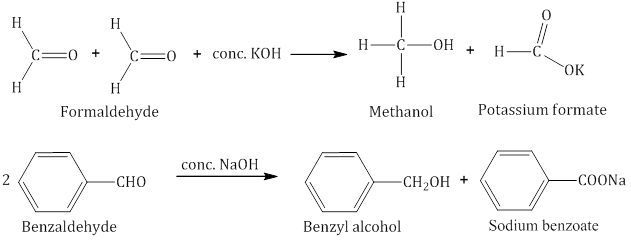
Test to distinguish aldehydes and ketones
- Tollen’s test: When an aldehyde is heated with Tollen’s reagent, it forms a silver mirror. Tollen’s reagent is ammoniacal solution of AgNO3.

Ketones do not form a silver mirror. - Fehling’s test: When an aldehyde is heated with Fehling’s reagent, it forms a reddish-brown precipitate of cuprous oxide.
Fehling’s reagent: Fehling solution A (aqueous solution of CuSO4) + Fehling solution B (alkaline solution of sodium potassium tartarate)
Ketones do not form a reddish-brown precipitate of cuprous oxide.
4. Reaction of carboxylic acids :
- Reactions involving cleavage of C–OH bond: Carboxylic acids on heating with mineral acids such as H2SO4 or with P2O5 give corresponding anhydride
- Anhydride formation:
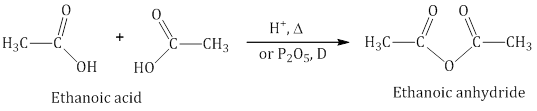
- Esterification: Carboxylic acids are esterified with alcohols in the presence of a mineral acid such as concentrated H2SO4 or HCl gas as a catalyst.
- Carboxylic acids react with PCl5, PCl3 and SOCl2 to form acyl chlorides.
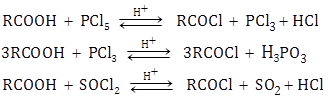
- Reaction with ammonia (NH3): Carboxylic acids react with ammonia to give ammonium salt, which on further heating at high temperature gives amides.
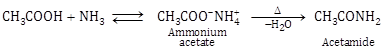
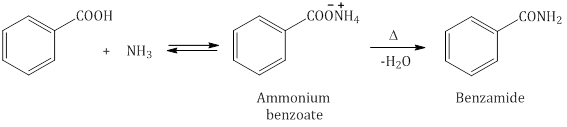
- Anhydride formation:
- Reactions involving COOH group:
- Reduction: Carboxylic acids are reduced to alcohols in the presence of LiAlH4 or B2H6.
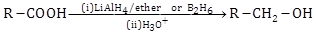
- Decarboxylation: Sodium or potassium salts of carboxylic acids on heating with soda lime (NaOH + CaO in ratio of 3:1) give hydrocarbons which contain one carbon less than the parent acid.
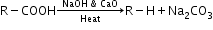
- Reduction: Carboxylic acids are reduced to alcohols in the presence of LiAlH4 or B2H6.
- Reactions involving substitution reaction in hydrocarbon part:
- Hell–Volhard–Zelinsky reaction: Carboxylic acids with an α-hydrogen are halogenated at the α-position on treatment with chlorine or bromine in the presence of a small amount of red phosphorus to give α-halocarboxylic acids).
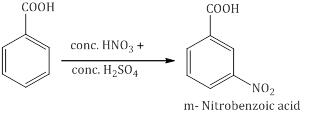
- Ring substitution in aromatic acids: Aromatic carboxylic acids undergo electrophilic substitution reactions. Carboxyl group in benzoic acid is electron-withdrawing group and is meta directing.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry