Equilibrium
Equilibrium, PDF Notes, Important Questions and Synopsis
SYNOPSIS
At equilibrium:
- Rate of forward reaction = rate of backward reaction
- Concentration (mole/litre) of reactant and product becomes constant.
- ΔG = 0
- Q = Keq
Law of mass action:
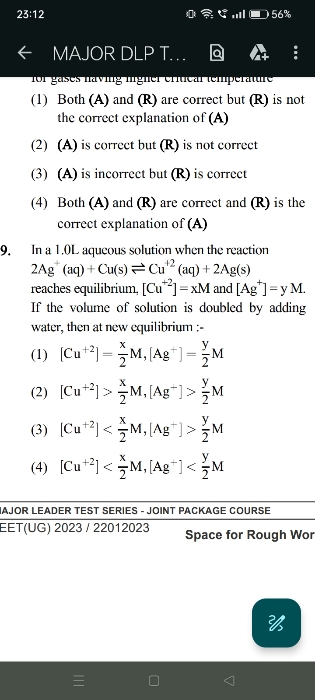
The rate at which a substance reacts is directly proportional to its active mass raised to suitable powers, and the rate of reaction is directly proportional to the product of all such active masses of reactants.
Factors influencing equilibrium constant (Kp or Kc):
- Stoichiometric representation of a reaction
- Mode of representing the change
- Temperature
- Units of pressure
- Units of Kp = (unit of pressure)∆n
- Units of Kc = (unit of concentration)∆n
- If Q = K, the reaction is in equilibrium.
- If Q > K, the reaction moves from right to left.
- If Q < K, the reaction moves from left to right.
Le Chatelier's Principle:
If a system at equilibrium is subjected to a disturbance or stress which changes any of the factors that determine the state of equilibrium, the system will react in such a way as to minimise the effect of the disturbance.
Effect of concentration:
- If the concentration of reactant is increased at equilibrium, then the reaction shifts in the forward direction.
- If the concentration of product is increased, then the equilibrium shifts in the backward direction.
- If the volume is increased, the pressure decreases; hence, the reaction will shift in the direction in which pressure increases,
i.e. in the direction in which the number of moles of gases increases and vice versa. - If the volume is increased, then for
Δn > 0, the reaction will shift in the forward direction
Δn < 0, the reaction will shift in the backward direction
Δn = 0, the reaction will not shift
Effect of pressure:
If the pressure is increased at equilibrium, then the reaction will try to decrease the pressure; hence, it will shift in the direction in which less number of moles of gases is formed.
Effect of inert gas addition:
- Constant pressure:
If the inert gas is added, then to maintain the pressure constant, volume is increased. Hence, the equilibrium will shift in the
direction in which larger number of moles of gas is formed.
Δn > 0, the reaction will shift in the forward direction
Δn < 0, the reaction will shift in the backward direction
Δn = 0, the reaction will not shift - Constant volume:
Inert gas addition has no effect at constant volume.
Effect of temperature:
Equilibrium constant is only dependent on the temperature.
If plot of lnK vs 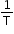 is plotted, then it is a straight line with slope =
is plotted, then it is a straight line with slope = 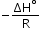 and intercept =
and intercept = 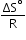
- For an endothermic (ΔH > 0) reaction, the value of the equilibrium constant increases with increase in temperature.
- For an exothermic (ΔH < 0) reaction, the value of the equilibrium constant decreases with increase in temperature.
- For ΔH > 0, the reaction shifts in the forward direction with increase in temperature.
- For ΔH < 0, the reaction shifts in the backward direction with increase in temperature.
- If the concentration of reactant is increased at equilibrium, then the reaction shifts in the forward direction.
- If the concentration of product is increased, then equilibrium shifts in the backward direction.
Ionisation: Separation of ions either on fusion or dissolution.
Strong electrolyte: A strong electrolyte forms a solution in which the solute is present almost entirely as ions.
Weak electrolyte: A weak electrolyte forms a solution in which the solute is incompletely ionised in solution.
Factors affecting degree of dissociation:
- Nature of solute: Strong electrolytes have degree of dissociation near 1 and weak electrolytes have degree of dissociation less than 1.
- Nature of solvent: Solvent with high dielectric constant shows higher degree of dissociation.
- Dilution: Degree of dissociation increases with dilution of solution.
- Temperature: Degree of dissociation increases with an increase in temperature.
- Addition of other species: Addition of another solute having an ion common to that of a weak electrolyte shows a decrease in the degree of dissociation of the weak electrolyte.
- Arrhenius acid: Substance which releases H+ ions in aqueous solutions.
- Arrhenius base: Substance which releases OH- ions in aqueous solutions.
- According to Arrhenius, water is amphoteric because it releases both H+ and OH- ions in aqueous
- Bronsted acid: Proton donor which donates H+ ions in aqueous solutions.
- Bronsted base: Proton acceptor which accepts H+ ions in aqueous solutions.
- According to Bronsted–Lowry, water is amphoteric because it donates as well as accepts protons.
- A Lewis acid is an electron pair acceptor.
- A Lewis base is an electron donor.
- According to Lewis concept, water behaves as a base.
- Mineral acids (H2SO4, HNO3, HCl) are strong acids.
- All organic acids (except those having the SO3H group) are weak acids.
Strong and weak bases:
Hydroxides of IA and IIA groups (i.e. alkali metals and alkaline earth metals) are strong bases [except Be(OH)2] and the rest of the hydroxides are weak bases.
Salts:
Simple salts: Salts formed by the neutralisation process are termed simple salts. These are of three types:
- Normal salts: Salts which do not contain a replaceable hydrogen atom or hydroxyl group. Examples: NaCl, NaNO3, K2SO4, Ca3(PO4)2, Na3BO3, Na2HPO3
- Acid salts: Salts which contain one or more replaceable hydrogen atoms. Examples: NaHCO3, NaHSO4, NaH2PO4
- Basic salts: Salts containing one or more hydroxyl groups. Examples: Zn(OH)Cl, Mg(OH)Cl, Fe(OH)2Cl
- Double salts: Addition compounds formed by the combination of two simple salts. Examples: Ferrous ammonium sulphate FeSO4.(NH4)2SO4.6H2O, potash alum K2SO4.Al2(SO4)3.24H2O
- Complex salts: These are formed by the combination of simple salts or molecular compounds. In this type of salts, the metal is in the central position with ligands. Examples: K4[Fe(CN)6], [Co(NH3)6]SO4
- Mixed salts: The salt which furnishes more than one cation (excluding H+) or more than one anion (excluding OH-) when dissolved in water. Examples: CaOCl2, NaKSO4
- pH is the negative logarithm of magnitude of H+ concentration.
- pH = -log[H+]
Simple buffers: A salt of a weak acid and a weak base in water. Examples: CH3COONH4, NH4CN, proteins and amino acids
Mixed buffers:
- Acidic buffer mixture: A weak acid with its conjugate base. (NaHCO3 + H2CO3,CH3COOH + CH3COONa)
- Basic buffer mixture: A weak base with its conjugate acid.
(NH4OH + NH4Cl)
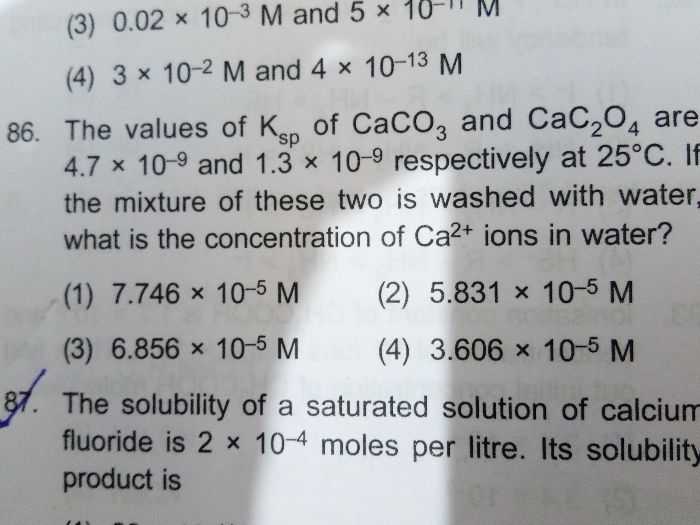
- If ionic product KI.P > KSP, precipitation occurs.
If KI.P = KSP, saturated solution (precipitation just begins or is just prevented).
- The process of salt hydrolysis is actually the reverse process of neutralisation.
- There are 4 types of salts:
- Salts of strong acid and weak base: NH4Cl, NH4NO3, CuSO4, FeCl3
- Salts of strong base and weak acid: KCN, CH3COONa, Na2S, HCOOK
- Salts of weak acid and weak base: CH3COONH4,(CH3COO)2Be, BeCO3, (NH4)3PO4
- Salts of strong acid and strong base: KNO3, NaCl, K2SO4
Acid–base indicator: An acid–base indicator is a substance whose colour changes with change in pH.
Ostwald theory:
- Indicators are either weak acids or weak bases.
- An indicator having acidic nature yields a coloured anion, while an indicator having basic nature yields a coloured cation in solution.
- All acid–base indicators are aromatic organic compounds which can exist in two tautomeric forms in equilibrium.
- One form is termed the benzoid form and the other is the quinonoid form.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry