Class 8 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 14: Measurement and Effects of Heat
Measurement and Effects of Heat Exercise Exercise
Solution 1.A

Solution 1.B
- The sentence is lying because the heat energy is measured in Joules.
- Heat flows from an object at higher temperature to an object at lower
temperature. - This sentence is telling truth. - Joule is the unit of heat. - This statement is telling the truth.
- Objects contract on heating. - This statement is lying. Object expands on heating.
- Atoms of a solid are free. - This statement is lying because the atoms of solid are not free
- The average kinetic energy of atoms in a hot object is less than the average kinetic energy of atoms in a cold object.
Solution 3.C
a. A thermometer is used to measure temperature.
b. The apparatus used to measure heat is called a calorimeter.
c. Temperature is the measure of the average kinetic energy of the atoms in a substance.
d. The heat contained in a substance is the measure of the total kinetic energy of atoms in the substance.
Solution 2
Shivani's tea will be prepared first because the intensity of flame provided by stove is high that the intensity of radiations given by solar cooker.
Solution 3
a.
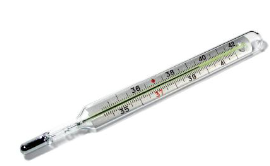
- Clinical thermometer has a narrow glass tube which has a bulb at the end. The bulb and part of the tube are filled with mercury.
- The bulb is kept in contact with an object whose temperature is to be measured.
- Normal human body temperature is 37°C (98.7°F).
- The clinical thermometer helps to measure the temperature between 35°C and 42°C.
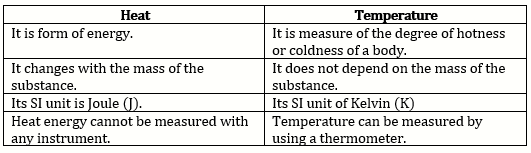
b. Difference between heat and temperature
c.
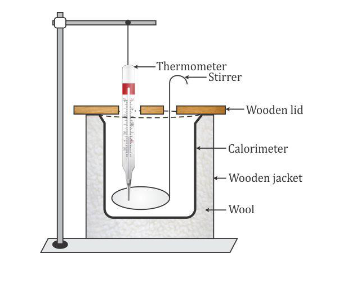
A calorimeter is a cylindrical vessel which is used to measure the amount of heat gained or lost by a body when it is mixed with another body.
It is made of thin copper sheets because
- Copper is a good conductor of heat, so the vessel soon acquires the temperature of its contents.
- Copper has low specific heat capacity, so the heat capacity of the calorimeter is low and the amount of heat energy taken up by the calorimeter from its contents to acquire the temperature of its contents is negligible.
d.
- They are not continuous. A small gap is kept between them at regular intervals.
- This is kept to accommodate the change in the length of the rails with change in temperature.
- If this gap is not kept, then the rails will get distorted due to expansion in summer which may lead to accidents.
e.
Expansion of liquids
A liquid does not have definite shape but has a definite volume. Thus, volumetric expansion of liquids is given by
V2 = V1 (1 + β × ∆T)
Here, β is volumetric expansion of liquids.
Expansion of gases
A gas does not have a fixed volume. It expands on heating, but if it is kept in a closed container, its volume cannot increase but its pressure increases.
If the gas is in a container with a movable piston, then the expansion of the gas is measured by keeping pressure constant. This volumetric expansion coefficient is called constant pressure expansion coefficient and is given by
V2 = V1 (1 + β × ∆T)
Here, β is volumetric expansion of gases.
Solution 4.a
Relation between Fahrenheit and Celsius scale is
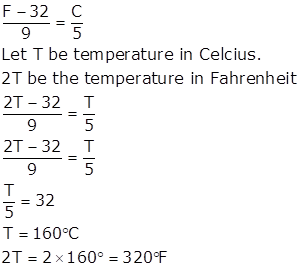
Solution 4.b
Length of iron rod = 20 m = 2000 cm at 18° C
Distance between the length of two rods = 0.4 cm
Temperature coefficient of linear expansion of iron = 11.5 × 10-6 °C-1
The bridge will be in good shape till both rods expand by 0.2 cm as the temperature is increased.
Let at temperature T rod expand 0.2 cm i.e., total expansion is 0.4 cm
Using formula for linear expansion of solids,
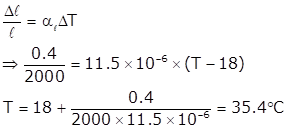
Solution 4.c
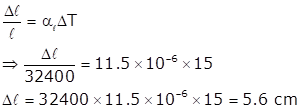
Solution 4.d
Let mass of B be M
Q = mc∆t
For body A,
Q = mc∆t
t = Q/mc … (1)
For body B,
4Q = M x 2c x t
M = 4Q/ 2c x T
From (1),
T = Q/mc
M = 4Q/ (2c x (Q/mc) = 2m
Solution 4.e
Let the specific heat capacity of substance be c.
Mass of substance, m = 3 kg = 3000 g
Heat given to substance, Q = 600 cal
Increase in temperature of substance = 10°C
The amount of heat in a body is given as,
Q = mc∆t
c = Q/(m∆t) = 600/ (3000 x 10) = 0.02 cal/g/°C
