Organic Compounds Containing Nitrogen
Organic Compounds Containing Nitrogen PDF Notes, Important Questions and Synopsis
SYNOPSIS
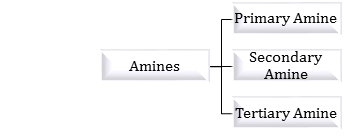
Classification:
Preparation of Amines:
Amines can be prepared using the following methods:
- Reduction of Nitro Compounds
Nitro compounds on reduction with hydrogen gas in the presence of finely divided nickel. palladium or platinum and also on reduction with metals in acidic medium give amines.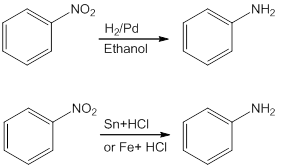
- Ammonolysis (Hoffmann’s method)
The process of cleavage of the C-X bond by ammonia molecule is known as ammonolysis.
The order of reactivity of halides with amines is RI >RBr > RCl -
Reduction of Nitriles
Nitriles on reducing with LiAlH4 or catalytic hydrogenation produce primary amines.
This method is used for preparing amines containing one carbon atom more than the starting amine. -
Reduction of Amides
Amides on reducing with LiAlH4 yield amines. -
Gabriel pthalimide synthesis
This method is used for the preparation of primary amines.
Pthalimide on reacting with ethanolic solution of KOH forms potassium salt of pthalimide which on heating with alkyl halide followed by alkaline hydrolysis yields the corresponding primary amine. -
Hoffmann bromamide degradation reaction
In this method, primary amines are prepared by treating an amide with bromine in an aqueous or ethanolic solution of NaOH.
In this degradation reaction,migration of alkyl or aryl group takes place from carbonyl carbon of the amide to the nitrogen atom.
The amine formed has one carbon atom less than the starting amide.
Reaction of Amines -
Acylation reaction
- The process of introducing an acyl group (R–CO–) into the molecule is called acylation.
- The reaction is carried out in the presence of a stronger base than the amine, such as pyridine, which removes HCl formed and shifts the equilibrium to the product side.
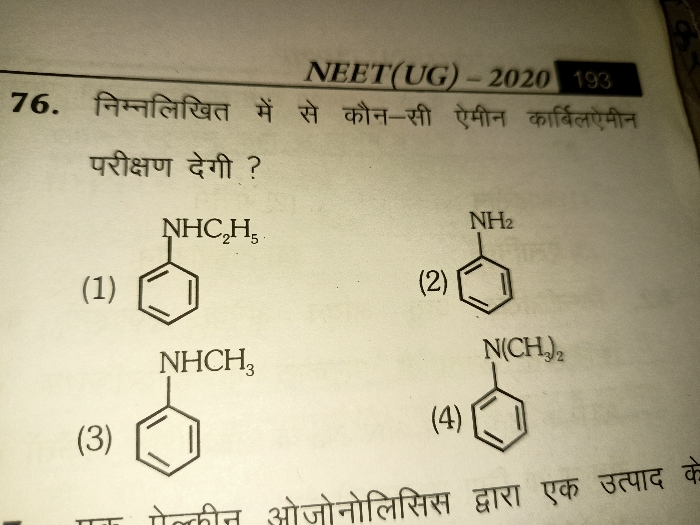
- Carbylamine reaction
- On heating aliphatic and aromatic primary amines with chloroform and ethanolic KOH they form isocyanides or carbylamines which have foul odour.
- Secondary and tertiary amines do not show this reaction. This reaction is used as a test for primary amines.
- Reaction with Nitrous acid
- Primary aliphatic amines react with nitrous acid to form aliphatic diazonium salts.
- Aromatic amines on treating with nitrous acid at low temperatures to form diazonium salts which are used in the synthesis of a variety of aromatic compounds.
- Secondary and tertiary amines react with nitrous acid in a different manner.
- Reaction with arylsulphonyl chloride
Hinsberg’s reagent or benzenesulphonyl chloride (C6H5SO2Cl) reacts with primary amines and secondary amines to form sulphonamides.
- Primary amine reacts with benzenesulphonyl chloride to form N-ethylbenzenesulphonyl amide.
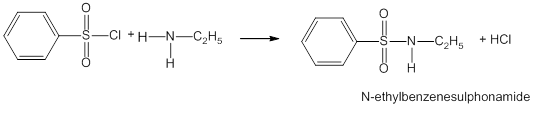
- With secondary amine,N,N-diethyl-benzenesulphonamide is formed.
N, N-diethylbenzene sulphonamide does not contain any H atom attached to nitrogen atom so it is not acidic and is therefore insoluble in alkali. - Tertiary amines do not react with benzenesulphonyl chloride.
The reaction of amines with benzenesulphonyl chloride in a different manner is used for the distinction of primary, secondary and tertiary amines.
-
Electrophilic substitution
Ortho- and para-positions to the -NH2 group become centres of high electron density.
So -NH2 group is ortho and para directing and a powerful activating group.
- Bromination
- Aniline reacts with bromine water at room temperature to give a white precipitate of 2, 4, 6-tribromoaniline.
- Aniline reacts with bromine water at room temperature to give a white precipitate of 2, 4, 6-tribromoaniline.
- Nitration
- Nitric acid is a nitrating agent plus a good oxidising agent. So direct oxidation of aromatic amines is not useful since it gives tarry oxidation products along with some nitro derivatives.
- In strong acidic medium, aniline is protonated to form the anilinium ion which is meta directing. Hence besides the ortho and para derivatives, significant amount of meta derivative is also formed.
- Sulphonation
Aniline on reacting with sulphuric acid forms anilinium hydrogen sulphate which on heating with sulphuric acid at 453-473K gives p-aminobenzene sulphonic acid as the major product.
- Reactions involving displacement of Nitrogen
Diazonium group is a very good leaving group and is substituted by other groups such as Cl-, Br-,I-,CN- and OH- which displace nitrogen from the aromatic ring. The nitrogen released from the reaction mixture escapes as a gas.
- Replacement by halide or cyanide ion:
This reaction is called Sandmeyer reaction in which nucleophiles like Cl-,Br- and CN- can be easily introduced in the benzene ring in the presence of Cu(I) ion.
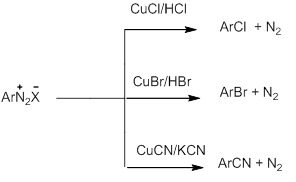
Alternatively, chlorine or bromine can also be introduced in the benzene ring by treating the diazonium salt solution with corresponding halogen acid in the presence of Cu powder. This is referred as Gatterman reaction.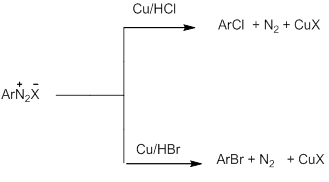
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry