Environmental Chemistry
Environmental Chemistry PDF Notes, Important Questions and Synopsis
SYNOPSIS
- Environmental pollution: It is the effect of undesirable changes in our surroundings that have harmful effects on plants, animals and human beings.
- Environmental pollution is of three types:
- Atmospheric pollution.
i. Tropospheric pollution.
ii. Stratospheric pollution. - Water pollution.
- Soil and land pollution.
- Atmospheric pollution.
- Tropospheric pollution: Tropospheric pollution occurs because of two types of pollutants:
- Gaseous air pollutants: These are oxides of sulphur, nitrogen, carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
- Particulate pollutants: Particulate pollutants are the minute solid particles or liquid droplets in the air. These are present in vehicle emissions, smoke particles from fires, dust particles and ash from industries. Examples of particulate pollutants are dust, mist, fumes, smoke, smog etc.
- Major air pollutants:
- Oxides of sulphur as pollutants:
Sources: Burning of fossil fuels containing sulphur.
Harmful effects:
These pollutants cause respiratory diseases such as asthma, bronchitis and emphysema in humans.
Sulphur dioxide causes irritation to the eyes, resulting in tears and redness of the eyes.
High concentration of sulphur dioxide leads to stiffness of flower buds which eventually fall off from plants.
Oxides of nitrogen as pollutants:
Sources:- At high altitudes, when lightning strikes, dinitrogen and dioxygen combine to form oxides of nitrogen.
- On burning fossil fuels in an automobile engine at a high temperature, dinitrogen and dioxygen combine to yield significant quantities of nitric oxide (NO) and nitrogen dioxide (NO2).
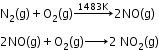
Harmful effects: - Damage the leaves of plants and retard the rate of photosynthesis.
- Nitrogen dioxide is a lung irritant that can lead to an acute respiratory disease in children.
- It is also toxic to living tissues.
- Nitrogen dioxide is also harmful to various textile fibres and metals
- Hydrocarbons as pollutant:
Source: Incomplete combustion of fuel used in automobiles.
Harmful effects:
- Hydrocarbons are carcinogenic, i.e. they cause cancer.
- They harm plants by causing ageing, breakdown of tissues and shedding of leaves, flowers and twigs
- Oxides of carbon as pollutant:
- Carbon monoxide
Source:- Incomplete combustion of carbon in coal, firewood, petrol, etc.
- By automobile exhaust.
Harmful effects:
It is highly poisonous to living beings because of its ability to block the delivery of oxygen to the organs and tissues. It binds to haemoglobin to form carboxyhaemoglobin, which is about 300 times more stable than oxyhaemoglobin. In blood, when the concentration of carboxyhaemoglobin reaches about 3–4%, the oxygen carrying capacity of blood is greatly reduced. This oxygen deficiency results in causing headaches, weak eyesight, nervousness and cardiovascular disorders.
- Carbon dioxide
- Respiration.
- Burning of fossil fuels for energy.
- Decomposition of limestone during the manufacture of cement.
- Volcanic eruptions.
- Deforestation.
- Harmful effects:
Causes global warming.
- Carbon monoxide
- Hydrocarbons as pollutant:
- Oxides of sulphur as pollutants:
- Green house effect and Global warming
- Green house effect: About 75% of the solar energy reaching Earth is absorbed by the Earth’s surface, which increases its temperature. The rest of the heat radiates back to the atmosphere. Some of the heat is trapped by gases such as carbon dioxide, methane, ozone, chlorofluorocarbon compounds (CFCs) and water vapour in the atmosphere. Thus, they add to heating of the atmosphere. This causes global warming.
- Green house: In a greenhouse, visible light passes through the transparent glass and heats up the soil and plants. The warm soil and plants emit infrared radiations. Because glass is opaque to infrared (heat) radiations, it partly reflects and partly absorbs these radiations. This mechanism keeps the energy of the Sun trapped in the greenhouse.
- Global warming: An increase in the average temperature of the Earth’s atmosphere (especially a sustained increase that causes climatic changes) which may be caused by additional heat being trapped by the greenhouse gases.
-
Acid rain: Normally rain water has a pH of 5.6 due to the presence of H+ ions formed by the reaction of rain water with carbon dioxide present in the atmosphere.
i. Harmful for trees (horticulture) or crop plants (agriculture) as it dissolves and washes away nutrients needed for their growth.
Source: Burning of fossil fuels, which contain sulphur and nitrogenous matter, such as coal and oil in power stations and furnaces or petrol and diesel in motor engines produce sulphur dioxide and nitrogen oxides. SO2 and NO2 after oxidation and reaction with water are major contributors to acid rain because polluted air usually contains particulate matter that catalyses oxidation.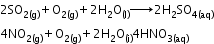
Harmful effects:
ii. Causes respiratory ailments in humans and animals.
iii. Affects plant and animal life in aquatic ecosystem when acid rain falls and flows as ground water to reach rivers, lakes etc.
iv. Corrodes water pipes resulting in leaching of heavy metals such as iron, lead and copper into drinking water.
v. Damages buildings and other structures made of stone or metal. Taj Mahal in India has been affected by acid rain. - Particulates in the atmosphere may be viable or non-viable:
- Viable are minute living organisms that are dispersed in the atmosphere.
- Examples: Bacteria, fungi, moulds, algae etc.
Non-viable particulates may be classified as
-
Smoke particulates: They consist of solids or mixture of solids and liquid particles formed during combustion of organic matter.
Examples: Cigarette smoke, smoke from burning of fossil fuel, garbage and dry leaves, oil smoke etc. -
Dust: They are composed of fine solid particles (over 1 μm in diameter) and produced during crushing, grinding and attribution of solid materials.
Examples: Sand from sand blasting, saw dust from wood works, pulverized coal, cement and fly ash from factories, dust storms etc. -
Mists: They are produced by particles of spray liquids and condensation of vapours in air. Examples: Sulphuric acid mist and mists from herbicides and insecticide.
-
Fumes: They are generally obtained by condensation of vapours during sublimation, distillation, boiling and several other chemical reactions. Generally, organic solvents, metals and metallic oxides form fume particles.
-
- Effect of air pollutants:
Smog: Smoke is a mixture of smoke, dust particles and small drops of fog.
Smog is of two types
Classical Smog
Photochemical Smog
1. It occurs in cool humid climate.
2. It is a mixture of smoke, fog and sulphur dioxide.
3. It is also called reducing smog.
1. It occurs in warm, dry and sunny climate.
2. Components of photochemical smog result from the action of sunlight on unsaturated hydrocarbons and oxides of nitrogen produced by automobiles and factories.
3. It is also called oxidizing smog.
- Effects of photochemical smog:
- Ozone and PAN act as powerful eye irritants.
- Ozone and nitric oxide irritate the nose and throat, and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing.
- Photochemical smog leads to cracking of rubber and extensive damage to plant life.
- It also causes corrosion of metals, stones, building materials, rubber and painted surfaces.
- Control of photochemical smog:
- Use of catalytic converters in automobiles, which prevent the release of nitrogen oxide and hydrocarbons to the atmosphere.
- Certain plants (e.g. Pinus, Juniparus, Quercus, Pyrus and Vitis) metabolise nitrogen oxide, and therefore their plantation could help in this matter.
- Stratospheric pollution is basically due to depletion of the ozone layer.
- Formation of ozone in stratosphere:
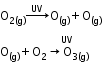
- Depletion of ozone layer by chlorofluorocarbons:
Release of chlorofluorocarbon compounds (CFCs), which are also known as freons, lead to their mixing with the normal atmospheric gases and eventually reach the stratosphere. - Effects of depletion of the ozone layer:
With the depletion of ozone layer, more UV radiation enters the troposphere.
UV radiations lead to
-
Ageing of skin, cataract, sunburn and skin cancer etc. in humans.
-
Killing of many phytoplanktons.
-
Damage to fish productivity.
-
Affect plant proteins which lead to the harmful mutation of cells.
-
Increases evaporation of surface water through the stomata of the leaves and decreases the moisture content of the soil.
Increase in the UV radiations damage paints and fibres, causing them to fade faster.
-
- Water pollution:
Major water pollutants
Sources
Harmful effects
Pathogens (Microorganisms)
Domestic sewage
Cause gastrointestinal diseases.
Organic wastes (leaves, grass and trash)
Domestic sewage, animal excreta and waste, decaying animals and plants and discharge from food processing factories
Lead to decrease in concentration of dissolved oxygen in water and lead to death of aquatic life.
Plant nutrients
Chemical fertilizers
Lead to a decrease in the amount of dissolved oxygen in the water which proves fatal for aquatic animals and plants
Toxic heavy metals (cadmium, mercury, nickel)
Industries and chemical factories
Can damage kidneys, central nervous system, liver etc.
Sediments
Erosion of soil by agriculture and strip mining
Harmful to humans, animals and plants as soil contains some amount of toxic metals
Pesticides (insecticides, herbicides and fungicides)
Chemicals used for killing insects, fungi and weeds
Lead to eutrophication.
Radioactive substances
Mining of uranium containing minerals
Radiation emitted is highly hazardous
Thermal pollutants
Water used for cooling in industries
Adversely affects aquatic life
- Biochemical Oxygen Demand (BOD): The amount of oxygen required by bacteria to break down the organic matter present in a certain volume of a sample of water.
- Eutrophication: The process in which nutrient enriched water bodies support a dense plant population, which kills animal life by depriving it of oxygen and results in subsequent loss of biodiversity.
- Pesticides: These are organic compounds which are used to protect plants from pests.
- Herbicides: They are used to kill weeds or undesirable vegetation. Examples: sodium chlorate (NaClO3) and sodium arsinite (Na3AsO3).
- Strategies to control environmental pollution:
-
Water management
Segregate the water as biodegradable and non-biodegradable waste:
Generated by thermal power plants which produce fly ash, integrated iron and steel plants which produce blast furnace slag and steel melting slag
Biodegradable waste:i. Generated by cotton mills, food processing units, paper mills, and textile factories.
ii. Management: They are deposited in landfills and are converted into compost.
Non-biodegradable water:
i. Generated by thermal power plants which produce fly ash, integrated iron and steel plants which produce blast furnace slag and steel melting slag
ii.Management: Recycling -
Toxic wastes are usually destroyed by controlled incineration.
-
Green chemistry: Green chemistry is a strategy to design chemical processes and products which reduces or eliminates the use and generation of hazardous substances. The chemical reactions should be such that the reactants are fully converted into useful environmental friendly products by using an environment friendly medium so that no chemical pollutants introduced in the environment.
-
- Green chemistry in daily life:
Purpose
Earlier
Now
Dry cleaning of clothes
<span >Tetrachloroethene (Cl2C=CCl2) which contaminates ground water
Liquefied carbon dioxide with a suitable detergent
Bleaching of paper
Chlorine gas
Hydrogen peroxide (H2O2) with suitable catalyst
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry

