Chemistry in Everyday Life
Chemistry in Everyday Life PDF Notes, Important Questions and Synopsis
SYNOPSIS
Drugs
- Drugs are chemicals of low molecular masses (~100 – 500u). These interact with macromolecular targets and produce a biological response. When the biological response is therapeutic and useful, these chemicals are called medicines and are used in diagnosis, prevention and treatment of diseases.
- Proteins which perform the role of biological catalysts in the body are called enzymes.
- The first function of an enzyme is to hold the substrate for a chemical reaction. Active sites of enzymes hold the substrate molecule in a suitable position, so that it can be attacked by the reagent effectively.
- The second function of an enzyme is to provide functional groups that will attack the substrate and carry out chemical reaction.
- Drugs inhibit the attachment of substrate on active site of enzymes in two different ways:
- Competitive inhibitors: Drugs compete with the natural substrate for their attachment on the active sites of enzymes. Such drugs are called competitive inhibitors.
- Non-competitive inhibitor: Some drugs do not bind to the enzyme’s active site. These bind to a different site of enzyme which is called allosteric site. This binding of inhibitor at allosteric site changes the shape of the active site in such a way that substrate cannot recognise it.
- Proteins which are vital for communication system in the body are called receptors. Receptors show selectivity for one chemical messenger over the other because their binding sites have different shape, structure and amino acid composition.
- In the body, message between two neurons and that between neurons to muscles is communicated through certain chemicals which are known as chemical messengers. They are received at the binding sites of receptor proteins. To accommodate a messenger, shape of the receptor site changes which brings about the transfer of message into the cell. Chemical messenger gives message to the cell without entering the cell.
Therapeutic Action of Drugs
Antacid -
Antacids are chemical substances which neutralises excess acid in gastric juices and give relief from acid indigestion, acidity, heart burns and gastric ulcers. Example: Eno, gelusil, digene etc.
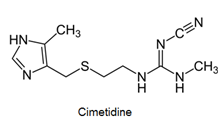
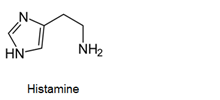
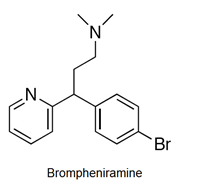
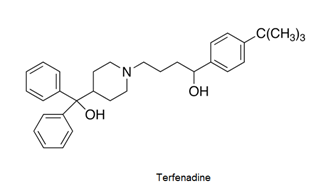
- Antihistamines are chemical substances which diminish the effect of histamine released in body and hence prevent allergic reactions. Example: brompheniramine (Dimetapp), terfenadine (Seldane)
- Antihistamines block the action of histamines and protect us from the allergy. Antihistamines work against symptoms of different kinds of allergies which includes hay fever and food allergy.
Neurologically Active Drugs
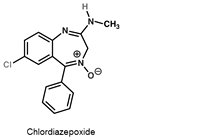
Tranquilizers
- Tranquilizers and analgesics are neurologically active drugs. They are the chemical compounds which are very useful in the treatment of stress and mild or severe mental diseases. They induce a sense of well-being in a person thereby releasing him from stress, tension, anxiety or irritability.
Analgesics
- Analgesics reduce the effect of pain without causing any mental confusion, paralysis or any other disturbances in the nervous system, so that you actually get rid of the pain without any imbalance in the nervous system.
- Analgesics can be broadly classified into two types:
- Non-narcotic analgesics
- Narcotic analgesics
- Non-narcotic analgesics: Aspirin and paracetamol belong to the class of non-narcotic analgesics. Aspirin inhibits the synthesis of chemicals known as prostaglandins which stimulate inflammation in the tissue and cause pain.
- Narcotic analgesics: These types of analgesic drugs are taken for medical use in prescribed doses, where they act by relieving the pain and producing sleep. These analgesics are chiefly used for the relief of postoperative pain, cardiac pain and pains of terminal cancer, and in child birth.
Antimicrobials
The different type of organisms like bacteria, fungi and virus are the main reason for infection and diseases. The drug used to prevent the pathogenicity of microorganism is called an antimicrobial agent. Examples: Antibiotics, antiseptics and disinfectants.
Antibiotics -
These are the substances which are derived from one microorganism to kill another microorganism. Antibiotics are useful against bacterial, fungal and parasitic infections but not against viral infections.
-
Examples: Ampicillin and amoxicillin.
-
There are two types of antibiotics which are as follows:
Bactericidal antibiotics: These antibiotics have killing effects on bacteria.
Example: Penicillin, Aminoglycosides, Ofloxacin.
Bacteriostatic antibiotics: These antibiotics have an inhibitory effect on bacteria.
Example: Erythromycin, Tetracycline, Chloramphenicol. -
Depends on the spectrum of action, antibiotics are further classified into three types. These are as follows:
Broad spectrum antibiotics: These antibiotics are widely used to kill or inhibit the Gram positive and Gram negative bacteria.
Example: Chloramphenicol
Narrow spectrum antibiotics: These antibiotics are widely effective against specific groups of bacteria.
Example: Penicillin G
Limited spectrum antibiotics: These antibiotics are effective against a single organism or a single disease.
Example: Dysidazirine.
Antiseptic and Disinfectants
- Antiseptics and disinfectants are the chemicals which either kill or prevent the growth of microorganisms.
- Examples are furacine, soframicine, etc.
- Antifertility Drugs
- Antifertility drugs are birth control pills which contain a mixture of synthetic estrogen and progesterone derivatives. Both of these compounds are hormones.
- It is known that progesterone suppresses ovulation. Synthetic progesterone derivatives are more potent than progesterone. Norethindrone is an example of synthetic progesterone derivative most widely used as antifertility drug.
Food additives: Food additives are the substances added to food to preserve its flavour or improve its taste and appearance.
Different types of food additives:
|
S. No. |
Name of food additive |
Examples |
|
1 |
Artificial sweetening agents: Chemical compounds which give a sweetening effect to food and enhance its flavour. |
Aspartame, Sucralose and Alitame |
|
2 |
Food preservatives: Chemical substances which are added to food material to prevent their spoilage due to microbial growth. |
Sugar, Salts, Sodium benzoate |
|
3 |
Food colours: Substances added to food to increase the acceptability and attractiveness of the food product. |
Allura Red AC, Tartrazine |
|
4 |
Nutritional supplements: Substances added to food to improve the nutritional value. |
Vitamins, minerals etc. |
|
5 |
Fat emulsifiers and stabilising agents: Substances added to food products to give texture and desired consistency. |
Egg yolk (where the main emulsifying chemical is lecithin) |
|
6 |
Antioxidants: Substances added to food to prevent oxidation of food materials. They help in the preservation of food. |
Butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) |
Cleansing Agents
Soaps and synthetic detergents are the two types of detergents which are used as cleansing agents. These improve cleansing properties of water which help in removal of fats that bind other materials to the fabric or skin.
Soaps
- Soaps are the detergents which are made up of sodium or potassium salts of long chain fatty acids.
Example: stearic, oleic and palmitic acids. - Soaps containing sodium salts are formed by heating fat (i.e., glyceryl ester of fatty acid) with aqueous sodium hydroxide solution. This reaction is known as saponification.
- On the basis of raw materials used there are different types of soaps.
- Types of soaps
S. No.
Descriptions
1.
Medicated soaps: These soaps are the soft soaps containing substances with medicinal properties. Neem soap and carbolic soaps are common examples of medicated soaps.
2.
Shaving soaps: These soaps are potassium sodium stearates and produce lasting lather. They contain glycerol to prevent rapid drying. A gum called rosin is added to these soaps which forms sodium rosinate which lathers well.
3.
Transparent soaps: These soaps are prepared by dissolving the soap in ethanol and then evaporating the excess solvent.
4.
Floating soaps: These soaps float in water and are prepared by beating tiny air bubbles into the product before it hardens.
5.
Soap chips: These are prepared by running a thin sheet of melted soap onto a cool cylinder and scrapping off the soaps in small broken pieces.
6.
Soap granules: These are dried miniature soap bubbles.
7.
Soap powder and scouring soaps: These substances contain some soap, a scouring agent (abrasive) such as powdered pumice or finely divided sand and builders such as sodium carbonate and trisodium phosphate. Builders help the soaps in its cleaning action.
Synthetic Detergents
Synthetic detergents are cleansing agents which have all the properties of soaps, but which actually do not contain any soap. These can be used both in soft and hard water as they give foam even in hard water. Some of the detergents give foam even in ice cold water. Synthetic detergents are mainly classified into three categories: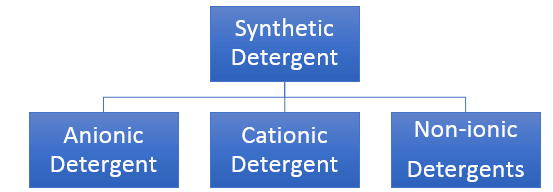
Anionic Detergents:
- Anionic detergents are sodium salts of sulphonated long chain alcohols or hydrocarbons. Alkyl hydrogensulphates formed by treating long chain alcohols with concentrated sulphuric acid are neutralised with alkali to form anionic detergents.
- In anionic detergents, the anionic part of the molecule is involved in the cleansing action. Sodium salts of alkylbenzenesulphonates are an important class of anionic detergents.
Cationic Detergent:
- Cationic detergents are quarternary ammonium salts of amines with acetates, chlorides or bromides as anions. Cationic parts possess a long hydrocarbon chain and a positive charge on nitrogen atom. Due to this they are called cationic detergents.
- Cetyltrimethylammonium bromide is a popular cationic detergent and is used in hair conditioners. Cationic detergents have germicidal properties and are expensive so they are used in limited amount.
Non-ionic Detergent:
Non-ionic detergents do not contain any ion in their constitution. One such detergent is formed when stearic acid reacts with polyethyleneglycol. Liquid dishwashing detergents are non-ionic type.
- Biodegradable detergents: Detergents with straight hydrocarbon chains which are easily decomposed by microorganisms.
Example: Sodium lauryl sulphate - Non-biodegradable detergents: Detergents with branched hydrocarbon chains which are not easily decomposed by microorganisms.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Principles Related to Practical Chemistry

