Biomolecules
Biomolecules PDF Notes, Important Questions and Synopsis
SYNOPSIS
Carbohydrates:
Carbohydrates may be defined as optically active polyhydroxy aldehydes or ketones or the compounds which produce such units on hydrolysis.
The most common sugar, used in our homes is named as sucrose whereas the sugar present in milk is known as lactose. Depending upon their behaviour on hydrolysis carbohydrates are classified into three groups.
Classification of Carbohydrates
- Classification of carbohydrates
- Monosaccharides
- Simplest carbohydrates
- Cannot be hydrolysed into simpler compounds
- Examples: Glucose, mannose
- Oligosaccharides
- Carbohydrates which give 2–10 monosaccharide units on hydrolysis
Examples: Sucrose, Lactose, Maltose
- Carbohydrates which give 2–10 monosaccharide units on hydrolysis
- Polysaccharides
- Carbohydrates which give a large number of monosaccharide units on hydrolysis
- Examples: Cellulose, starch
- Monosaccharides
Monosaccharides are further classified on the basis of the number of carbon atoms and the functional group present in them. If a monosaccharide contains an aldehyde group, it is known as an aldose and if it contains a keto group, it is known as a ketose.
Different Types of Monosaccharides
C-Atoms
Term
Aldehyde
Ketone
3
Triose
Aldotriose
Ketotriose
4
Tetrose
Aldotetrose
Ketotetrose
5
Pentose
Aldopentose
Ketopentose
6
Hexose
Aldohexose
Ketohexose
7
Heptose
Aldoheptose
Ketoheptose
Glucose (Aldohexose)
Glucose is the monomer for many other carbohydrates. Alone or in combination, glucose is probably the most abundant organic compound on the earth. Glucose occurs freely in nature as well as in the combined form. It is present in sweet fruits and honey.
Cyclic Structure of Glucose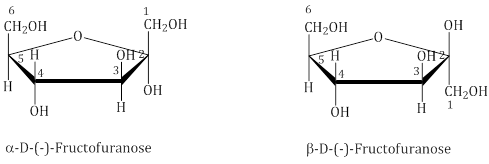
Structure of Fructose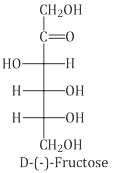
The cyclic structures of two anomers of fructose are represented by Haworth structures: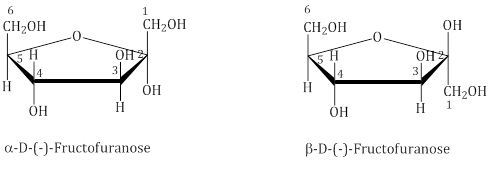
- Disaccharides
Disaccharides on hydrolysis with dilute acids or enzymes yield two molecules of either the same or different monosaccharides. The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage through oxygen atom is called glycosidic linkage.
Sucros
- These two monosaccharides are held together by a glycosidic linkage between C1 of α-glucose and C2 of β-fructose. Reducing groups of glucose and fructose are involved in glycosidic bond formation due to this sucrose is a non reducing sugar.
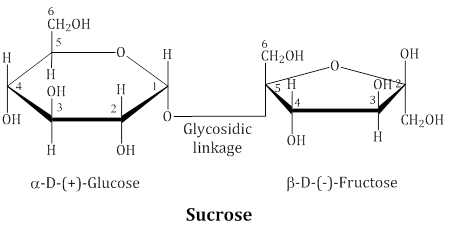
- Sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose. Thus, hydrolysis of sucrose brings about a change in the sign of rotation, from dextro (+) to laevo (–) and the product is named as invert sugar.
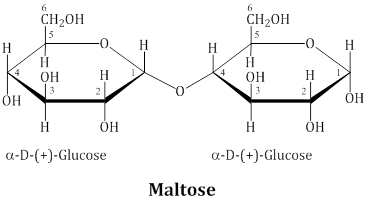
Maltose -
Maltose is composed of two α-D-glucose units in which C1 of one glucose (I) is linked to C4 of another glucose unit (II).
-
The free aldehyde group can be produced at C1 of second glucose in solution and it shows reducing properties so it is a reducing sugar.
- These two monosaccharides are held together by a glycosidic linkage between C1 of α-glucose and C2 of β-fructose. Reducing groups of glucose and fructose are involved in glycosidic bond formation due to this sucrose is a non reducing sugar.
- Polysaccharides
If a large number of monosaccharide units are joined together, we get polysaccharides. These are the most common carbohydrates found in nature. They have mainly one of the following two functions- either as food materials or as structural materials.
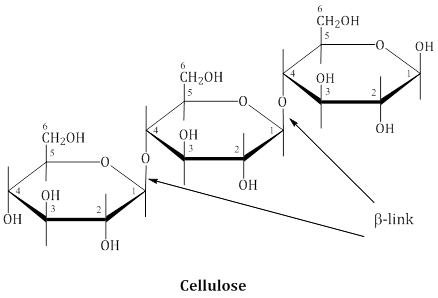
Cellulose
- Cellulose occurs exclusively in plants. It is a predominant constituent of cell wall of plant cells.
- Cellulose is a straight chain polysaccharide composed only of β-D-glucose units which are joined by glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
Proteins:
Proteins are classified on the basis of their chemical composition, shape and solubility into two major categories as discussed below.
- Simple Proteins
Simple proteins are those which on hydrolysis give only amino acids. According to their solubility, the simple proteins are further divided into two major groups—fibrous and globular proteins.
Fibrous Proteins: These are water-insoluble animal proteins, e.g. collagen (major protein of connective tissues), elastins (protein of arteries and elastic tissues) and keratins (proteins of hair, wool and nails) are good examples of fibrous proteins. Molecules of fibrous proteins are generally long and thread like.
Globular Proteins: These proteins are generally soluble in water, acids, bases or alcohol. Some examples of globular proteins are albumin of eggs, globulin (present in serum) and haemoglobin. Molecules of globular proteins are folded into compact units which are spherical.
- Conjugated Proteins
Conjugated proteins are complex proteins which on hydrolysis yield not only amino acids but also other organic or inorganic components. The non-amino acid portion of a conjugated protein is called a prosthetic group.
- Proteins can also be classified on the basis of the functions they perform:
Class
Functions
Examples
1
Transport Proteins
Transport of oxygen, glucose and other nutrients
Haemoglobin, lipoproteins
2
Nutrient and Storage Proteins
Store proteins required for the growth of embryo
Gliadin (wheat), Ovalbumin (egg),
Casein (milk)
3
Structural Proteins
Give biological structures,
strength or protection
Keratin (hair and nails),
collagen (cartilage)
4
Defence Proteins
Defend organisms against
invasion by other species
Antibodies, snake
venom
5
Enzymes
Act as catalysts in biochemical reactions
Trypsin, pepsin
6
Regulatory Proteins
Regulate cellular or
physiological activity
Insulin
- The actual structure of a protein can be discussed at four levels:
-
Primary structure: Information regarding the sequence of amino acids in a protein chain is called its primary structure. The primary structure of a protein determines its functions and is critical to its biological activity.
- Secondary structure: The secondary structure arises because of regular folding of the polypeptide chain due to hydrogen bonding between carbonyl and – NH– groups. Two types of secondary structures have been reported. These are α-helix when the chain coils up and a β-pleated sheet when hydrogen bonds are formed between the chains.
- Tertiary structure: It is the three-dimensional structure of proteins. It arises due to folding and superimposition of various α-helical chains or β-pleated sheets.
- Quaternary structure: The quaternary structure refers to the way in which simple protein chains associate with each other resulting in the formation of a complex protein.
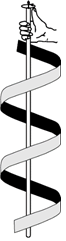
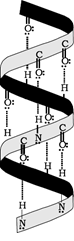
α-Helix structure of proteins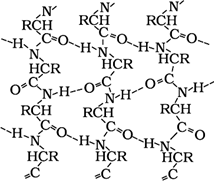
β-Pleated sheet structure of proteins
Enzymes:
Enzymes acts as a biological catalysts. In addition to the protein structure, most active enzymes are associated with some non-protein component required for their activity, called coenzymes. Example: Nicotinamide adenine dinucleotide (NAD) is a coenzyme which is associated with several dehydrogenation enzymes. Some important enzymes and their functions are given below:
|
|
Enzymes |
Reaction catalysed |
|
(i) |
Invertase or sucrase |
Sucrose → Glucose + fructose |
|
(ii) |
Maltase |
Maltose → Glucose + Glucose |
|
(iii) |
Lactase |
Lactose → Glucose + Galactose |
|
(iv) |
α-Amylase |
Starch → n Glucose |
|
(v) |
Emulsin |
Cellulose → n Glucose |
|
(vi) |
Urease |
NH2CONH2 → CO2 + 2NH3 |
|
(vii) |
Carbonic anhydrase |
H2CO3 → CO2 + H2O |
|
(viii) |
Pepsin |
Proteins → α-amino acids |
|
(ix) |
Trypsin |
Proteins → α-amino acids |
|
(x) |
Nucleases |
DNA or RNA → Nucleotides |
|
(xi) |
DNA polymerase |
Deoxynucleotide triphosphates → DNA |
|
(xii) |
RNA polymerase |
Ribonucleotide triphosphates → RNA |
Vitamins
Vitamins are organic compounds required in the diet in small amounts to perform specific biological functions for normal maintenance of optimum growth and health of the organism. Most of the vitamins cannot be synthesised in our body, but plants can synthesise almost all of them, so they are considered essential food factors.
Classification of Vitamins
Vitamins are classified into two groups depending on their solubility in water or fat.
|
Fat-soluble vitamins |
Water-soluble vitamins |
|
These vitamins are soluble in fat and oils but insoluble in water. |
These vitamins are soluble in water. |
|
They are stored in the liver and adipose (fat- storing) tissues. |
Water-soluble vitamins must be supplied regularly in the diet because they are readily excreted in urine and cannot be stored (except Vitamin B12) in our body. |
|
Examples: Vitamins A, D, E and K |
Examples: Vitamins B and C |
Important Vitamins, their Sources and their Deficiency Diseases
|
Name of vitamin |
Source |
Deficiency diseases |
|
Vitamin A |
Fish liver oil, carrots, butter and milk |
Xerophthalmia (hardening of the cornea of the eye) Night blindness |
|
Vitamin B1 (Thiamine) |
Yeast, milk, green vegetables and cereals |
Beriberi (loss of appetite, retarded growth) |
|
Vitamin B2 (Riboflavin) |
Milk, egg white |
Cheilosis (fissuring at the corners of the mouth and lips), digestive disorders and burning sensation of the skin |
|
Vitamin B6 (Pyridoxine) |
Yeast, milk, egg yolk, cereals and gram |
Convulsions |
|
Vitamin B12 |
Meat, fish, egg and curd |
Pernicious anaemia (RBC deficient in haemoglobin) |
|
Vitamin C (Ascorbic acid) |
Citrus fruits, amla and green leafy vegetables |
Scurvy (bleeding gums) |
|
Vitamin D |
Exposure to sunlight, fish and egg yolk |
Rickets (bone deformities in children) and osteomalacia (soft bones and joint pain in adults) |
|
Vitamin E |
Vegetable oils such as wheat germ oil, sunflower oil |
Increased fragility of RBCs and muscular weakness |
|
Vitamin K |
Green leafy vegetables |
Increased blood clotting time |
Nucleic acids:
- Nucleic acids are mainly of two types:
- Deoxyribonucleic acid (DNA)
- Ribonucleic acid (RNA)
- Chemical Composition of Nucleic Acids
- DNA or RNA on complete hydrolysis yields a pentose sugar, phosphoric acid and nitrogen containing heterocyclic compounds.
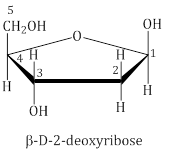
- In DNA molecules, the sugar moiety is β-D-2-deoxyribose.
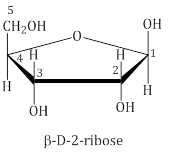
- In RNA molecule, the sugar moiety is β-D-2-ribose.
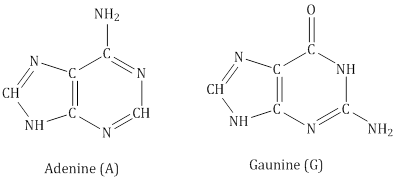
- DNA contains four bases:
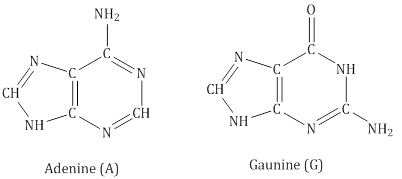
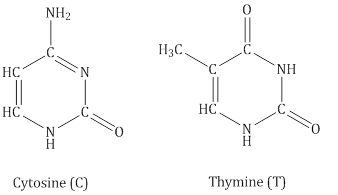
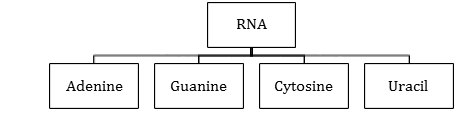
- RNA contains four bases:
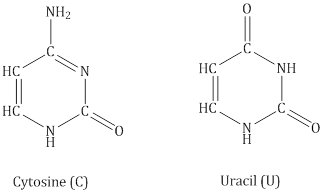
- Structure of Nucleic Acids
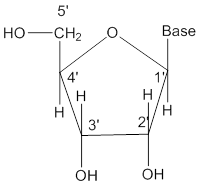
Nucleoside
- Nucleotide is a unit formed by linking a base to 1’ position of sugar.
- The sugar carbons are numbered as 1’, 2’, 3’ etc. in order to distinguish from the bases.
Nucleotide
- It is obtained when nucleoside is linked to phosphoric acid at 5’-position of sugar moiety.
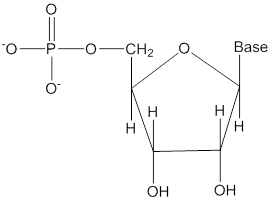
- Nucleotides are linked by phosphodiester linkage between 5’ and 3’ carbon atoms of the pentose sugar.
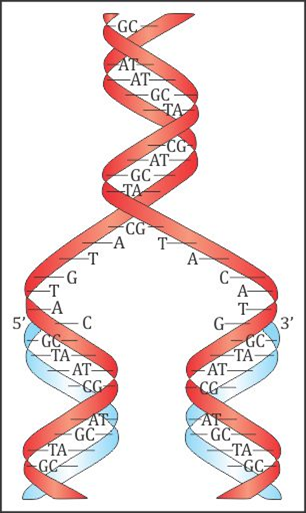
- James Watson and Francis Crick gave a double strand helix structure for DNA.
- In this two nucleic acids chains are wound about each other and held together by hydrogen bonds between pairs of bases.
- The two strands are complimentary to each other because the hydrogen bonds are formed between specific pairs of bases.
- Adenine forms hydrogen bonds with thymine whereas cytosine forms hydrogen bondsith guanine.
RNA
- In secondary structure of RNA, helices are present which are only single stranded.
- They sometimes fold back on themselves to form a double helix structure.
- RNA molecules are of three types and they perform different functions.
- They are named as:
- Messenger RNA (m-RNA)
- Ribosomal RNA(r-RNA)
- Transfer RNA (t-RNA)
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry

