ICSE Class 10 - Atomic Mass and Molecular Mass Videos
Mole Concept and Stoichiometry
Mole Concept and Stoichiometry, Atomic Mass and Molecular Mass
- The mass of 5.6~dm^{3} of a certain gas at STP is 12-0 g. Calculate the relative molecular mass of the gas.
- what is formula of law of construction of mass
- Calculate the minority of 90% (wt/v) H2SO4 having density 1.98g/mol
-
please solve the (v) part.

- What is Gram Molecular Mass
- What is Gram Molecular Mass and how its is calculated?
- The mass of an atom of lead on=202
-
Pls solve it.
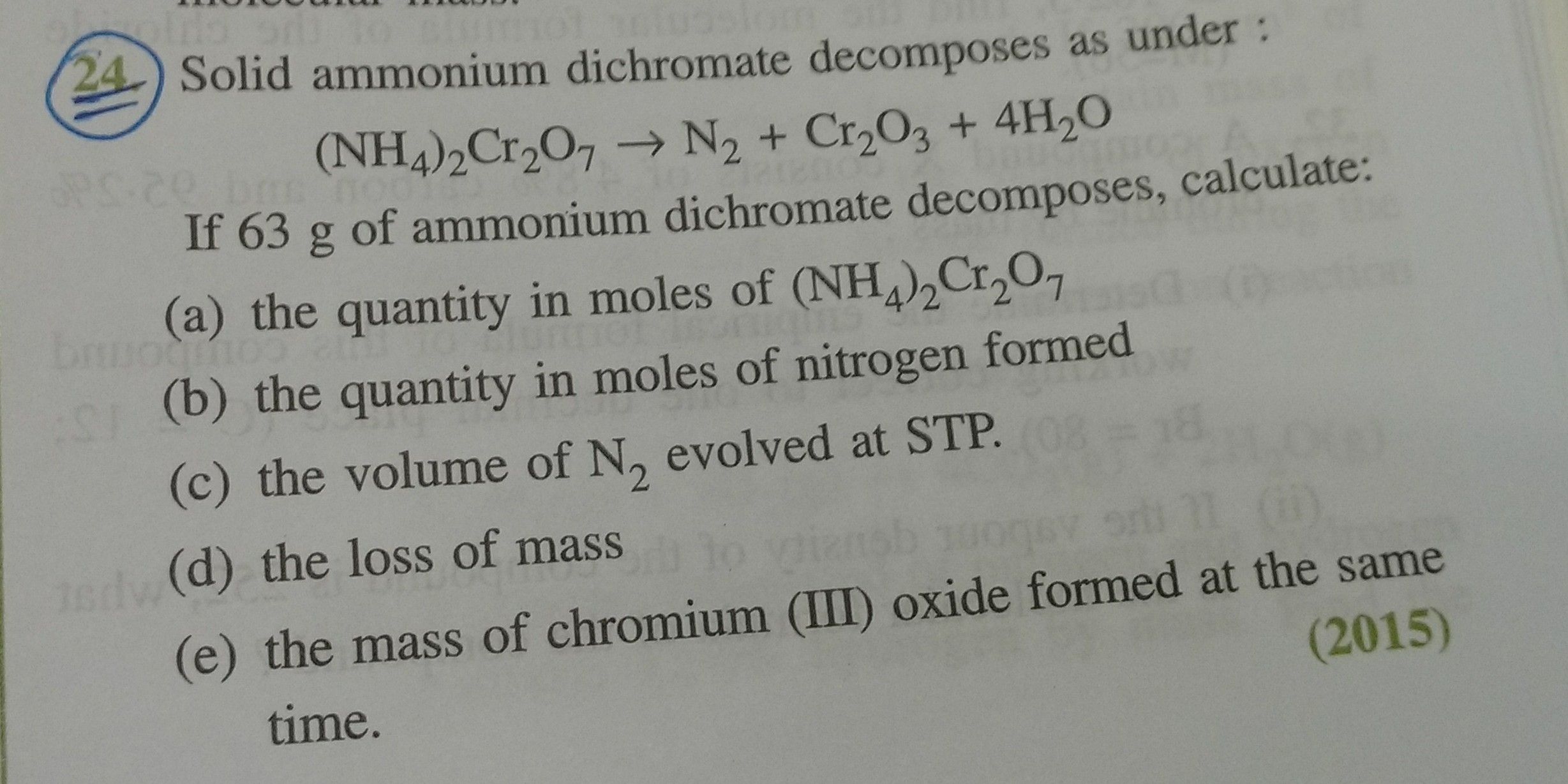
- Calculate the relative molecular mass[molecular weight] of 290 ml of a gas A at 17 degrees celsius and 1520 mm pressure which weighs 2.73 gram at S.T.P.
- A compound of emperical formula CH2O has a VDof 30 write down its molecular formula.

