ICSE Class 10 Physics Specific Heat Capacity
- In the principle of caloriemetry we have to assume that there is no loss of heat but it is not the same practically is the law of conservation violated in this case??
-
ch. heat numerical 8
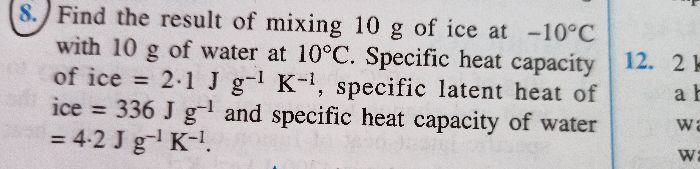
-
exercise 11 b q 8
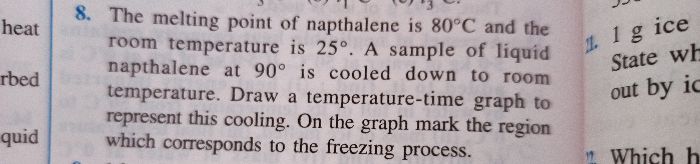
- Why inorder to measure specific heat capacity we consider unit mass only and why is it not dependent on mass?
- What is the meaning of the following: When two bodies are placed in contact, the total amount of heat is equal to the sum of heat of individual body.
- A Calorimeter of mass 50 grams and specific heat capacity 0.42 J/ g ℃ contains some mass of water at 20 ℃. A metal piece of mass 20 grams at 100℃ is dropped into the calorimeter. After stirring, the final temperature is found to be 22℃. Find the mass of water used in the calorimeter. (Specific Heat capacity of metal piece: 0.3 J/g ℃ Specific Heat capacity of water: 4.2 J/g ℃)
- how can i solve calorimetry numerocals
- si unit of energy
- IF A HEATER WITH POWER 1000W GIVES 3.6*10^6 joules OF HEAT ENERGY IN ONE HOUR.WHAT WILL BE THE TEMPERATURE RAISED IN THE 50KG OF WATER TEMPERATURE 30 DEGREE CELCIUS BY THAT HEAT.


