Class 10 FRANK Solutions Chemistry Chapter 9 - B - Nitric Acid
Get complete access to Frank Solutions for ICSE Class 10 Chemistry Chapter 9 B − Nitric Acid at TopperLearning. Learn to explain Ostwald’s process to prepare nitric acid along with a labelled diagram. Revise the properties and applications of nitric acid with our detailed Chemistry solutions.
In this ICSE Class 10 Chemistry chapter, you will also practise the balancing of certain Chemistry equations. For your board exam, you can prepare MCQs and various short answer questions by revising our textbook solutions and by attempting our online practice tests. You can check our Chemistry doubts and solutions, concept videos and question papers too.
Nitric acid Exercise 235
Solution 1

Solution 2

Solution 3

Nitric acid Exercise 236
Solution 4

Solution 5

Solution 6
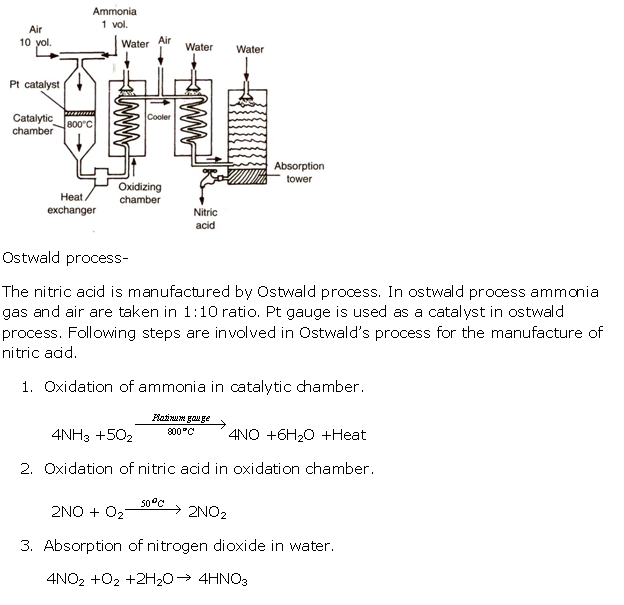
Solution 7

Solution 8

Solution 9

Solution 10

Solution 11

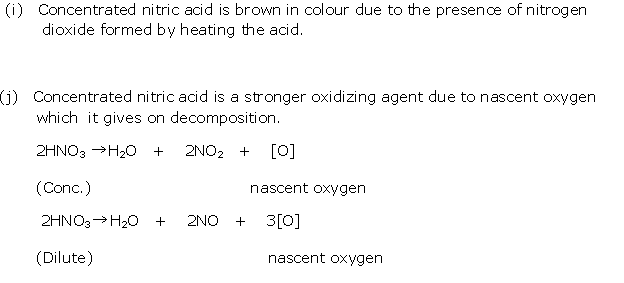
Solution 12

Solution 13
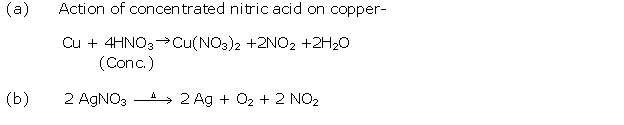
Solution 14

Solution 15
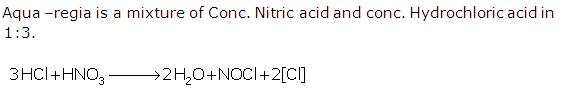
Solution 16

Solution 17

Nitric acid Exercise 237
Solution 18
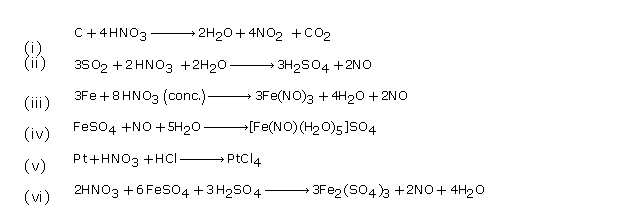
Solution 19
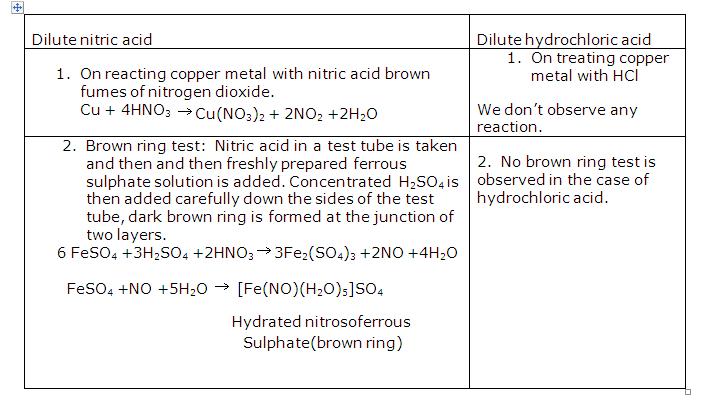
Solution 20
(i) NO2
(ii) H3PO4
(iii) Magnesium
(iv) Platinum gauze
Solution 21
(i) Aqua regia is a mixture of nitric acid and hydrochloric acid.
(ii) Fuming nitric acid is obtained by dissolving excess of nitrogen oxide in conc. nitric acid.
(iii) 98% nitric acid is obtained by distilling 68% nitric acid with conc. H2SO4 under pressure.
(iv) Ammonal is a mixture of ammonium nitrate and aluminium powder.
(v) 3Cu + 8HNO3 (dilute) → 3Cu(NO3)2 + 4H2O + 2NO
Solution 1991-1

Solution 1991-2
Solution 1991-3
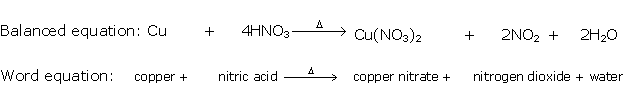
Solution 1992-1

Solution 1992-2
Solution 1992-3

Nitric acid Exercise 238
Solution 1992-4
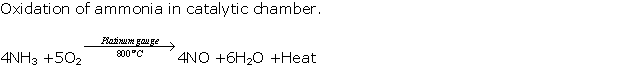
Solution 1994-1
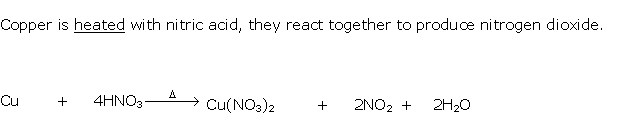
Solution 1994-2
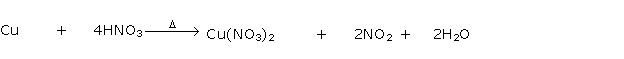
Solution 1994-3

Solution 1994-4
Solution 1995-1
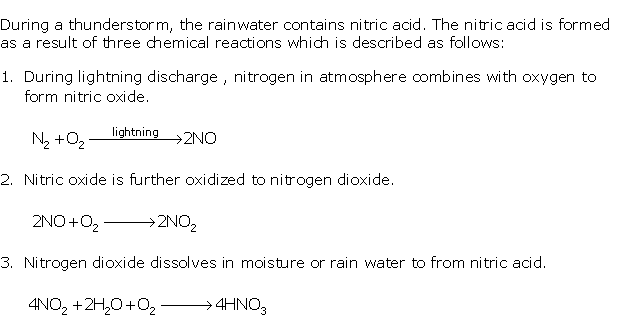
Solution 1997-1
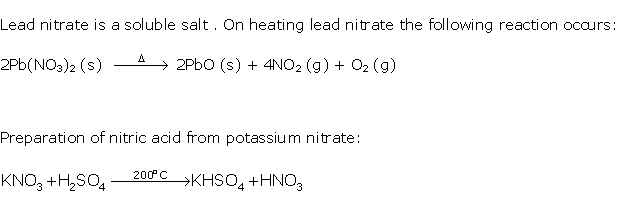
Solution 1998-1
Solution 1999-1
When concentrated nitric acid is added to copper brown fumes of nitrogen dioxide are observed.
Solution 2000-1
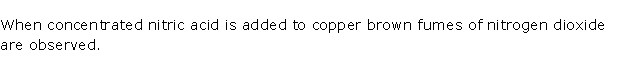
Solution 2001-1
(i) Nitrogen dioxide
(ii) Ammonia gas
Solution 2001-2
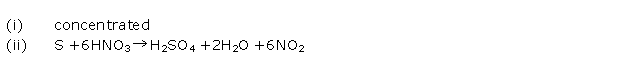
Solution 2001-3
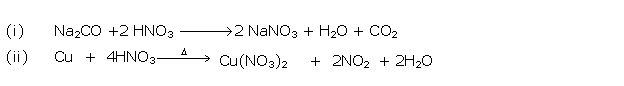
Solution 2002-1
Solution 2002-2

Nitric acid Exercise 239
Solution 2002-3
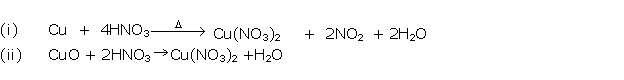
Solution 2002-4
Solution 2003-1

Solution 2003-2
Solution 2005-1
Solution 2005-2
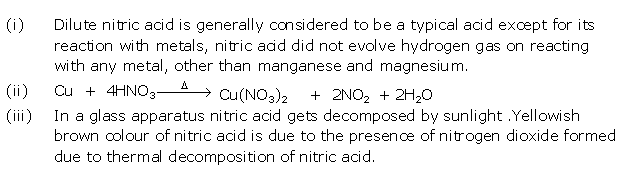
Solution 2006-1

Solution 2007-1
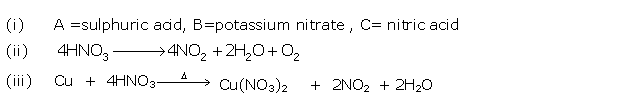
Solution 2007-2
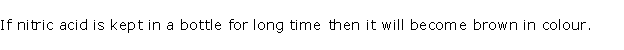
Nitric acid Exercise 240
Solution 2008-1

Solution 2008-2
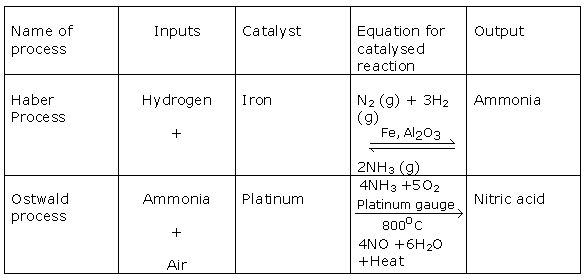
Solution 2009-1

Solution 2011-1
(i) The complete apparatus should be made of glass only.
(ii) At high temperature, nitric acid decomposes and the glass apparatus may get damaged. Sodium formed at a higher temperature forms a hard crust which sticks to the walls of the retort.
Solution 2011-2
Brown fumes of nitrogen dioxide are produced. Copper reacts with concentrated nitric acid to produce copper nitrate, water and nitrogen dioxide.
Solution 2013-1
(i) When sulphur is treated with conc. nitric acid, it produces nitrogen dioxide.
(ii) ![]()
Solution 2015-1
(i) Dilute nitric acid is generally considered a typical acid except for its reaction with metals because it does not liberate hydrogen. It is a powerful oxidising agent, and nascent oxygen formed oxidises hydrogen in water.
(ii) Although pure concentrated nitric acid is colourless, it appears yellow when left standing in a glass bottle due to the dissolution of reddish brown nitrogen dioxide gas in the acid. Nitrogen dioxide is produced because of the thermal decomposition of a portion of nitric acid.
4HNO3 → 2H2O + 4NO2 + O2
(iii) An all-glass apparatus is used in the laboratory preparation of nitric acid because nitric acid vapour corrodes rubber and cork.
Solution 2016-1
(i) Cold, dilute nitric acid reacts with copper to give nitric oxide.
(ii) Hot, concentrated nitric acid reacts with sulphur to form nitrogen dioxide.
Solution 2017-1
(i) 3Cu + 8HNO3→ 3Cu(NO3)2 + 4H2O+ 2NO↑
(ii) ![]()
(iii) ![]()
(iv) ![]()

