write the short note on the folowing purification method with suitable examples
1) Distillation 2) sublimation
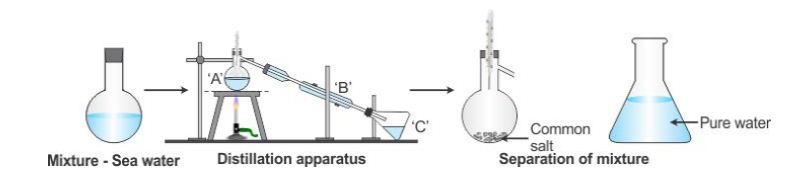
- Distillation
Distillation is the method of getting a pure liquid from a solution by evaporation and then condensing the vapour of the liquid having a low boiling point.
When a solution is heated, the liquid component of the mixture evaporates in the form of vapour. The vapour then recondenses into the liquid form which is pure and is called a distillate.
The advantage of this method is that both components of the solid-liquid mixture are obtained.
Example: Distillation of sea water to give pure water in the laboratory
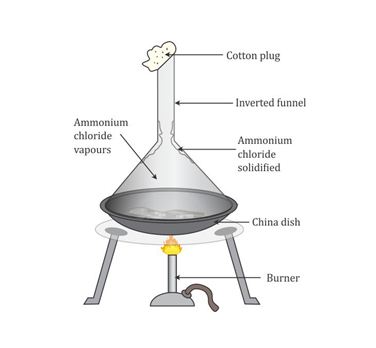
- Sublimation
The process in which a solid change directly into its vapour on heating is called sublimation.
This method is based on the difference between the sublimable and non-sublimable nature of solids. The mixture of sublimable and non-sublimable substances is heated in an evaporating dish covered with an inverted funnel.
Example: Separation of ammonium chloride from sodium chloride in the laboratory
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Classification of Elements and Periodicity in Properties
- 2 Chemical Bonding and Molecular Structure
- 3 States of Matter
- 4 Thermodynamics
- 5 Equilibrium
- 6 Hydrogen
- 7 Hydrocarbons
- 8 Environmental Chemistry
- 9 Solutions
- 10 Chemical Kinetics
- 11 Surface Chemistry
- 12 Biomolecules
- 13 Polymers
- 14 Chemistry in Everyday Life
- 15 Gravitation
- 16 Sequences and Series
- 17 Mathematical Reasoning
- 18 Differential Equations
- 19 Three Dimensional Geometry
- 20 Electromagnetic Waves
- 21 Communication Systems
- 22 Complex Numbers and Quadratic Equations
- 23 Permutations and Combinations
- 24 Laws of Motion
- 25 Current Electricity
- 26 Work, Energy and Power
- 27 Atomic Structure
- 28 Kinematics
- 29 Matrices and Determinants
- 30 Vector Algebra
- 31 Trigonometry
- 32 Integral Calculus
- 33 Chemical Thermodynamics
- 34 Redox Reactions and Electrochemistry
- 35 p-Block Elements
- 36 d - and f - Block Elements
- 37 Some Basic Principles of Organic Chemistry
- 38 Organic Compounds Containing Halogens
- 39 Organic Compounds Containing Oxygen
- 40 Organic Compounds Containing Nitrogen
- 41 Sets, Relations and Functions
- 42 Limit, Continuity and Differentiability
- 43 Physics and Measurement
- 44 Rotational Motion
- 45 Properties of Solids and Liquids
- 46 Kinetic Theory of Gases
- 47 Oscillations and Waves
- 48 Electrostatics
- 49 Magnetic Effects of Current and Magnetism
- 50 Electromagnetic Induction and Alternating Currents
- 51 Optics
- 52 Dual Nature of Matter and Radiation
- 53 Atoms and Nuclei
- 54 Electronic Devices
- 55 Co-ordination Compounds
- 56 Purification and Characterisation of Organic Compounds
- 57 Statistics and Probability
- 58 s-Block Element (Alkali and Alkaline Earth Metals)
- 59 Solid State
- 60 Some Basic Concepts in Chemistry
- 61 General Principles and Processes of Isolation of Metals
- 62 Co-ordinate Geometry
- 63 Mathematical Induction
- 64 Binomial Theorem and its Simple Applications
- 65 Principles Related to Practical Chemistry