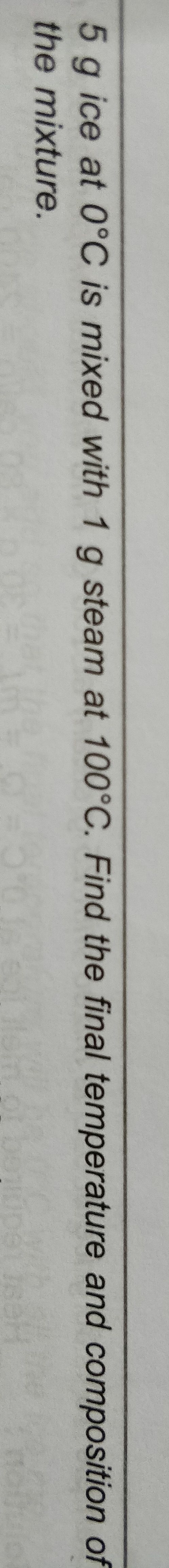NEET Class neet Answered
Why Q=0 is taken?
Asked by patra04011965 | 19 Mar, 2019, 01:17: PM
When a thermodynamic system in not making any transaction of heat either it's not taking any heat or removal of any heat from its surrounding the the value of Q becomes zero. Which means internal energy of system is directly changing to work done, or work done on the system is fully changing to its internal energy .
Answered by Ankit K | 19 Mar, 2019, 02:12: PM
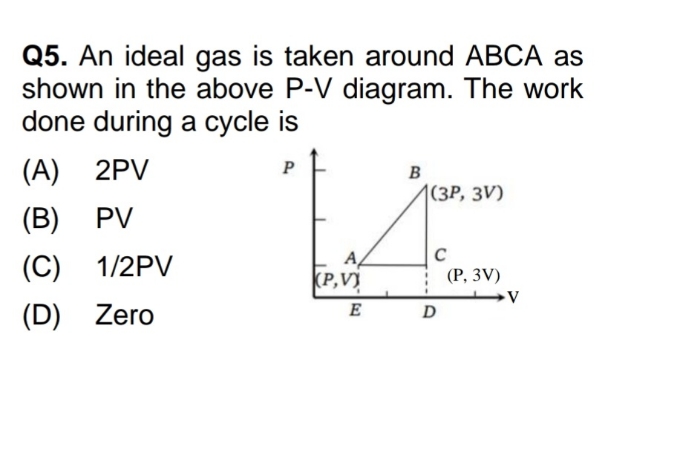
NEET neet - Physics
Asked by yadavaradhana9335 | 19 Feb, 2024, 04:53: PM
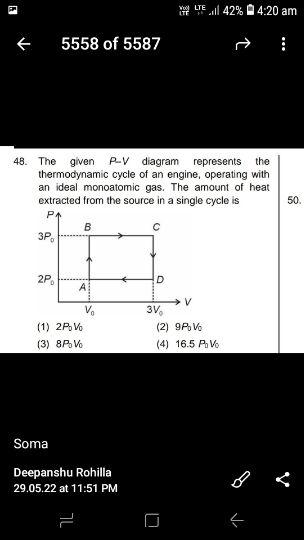
NEET neet - Physics
Asked by sujitjana971 | 18 Dec, 2022, 05:23: PM
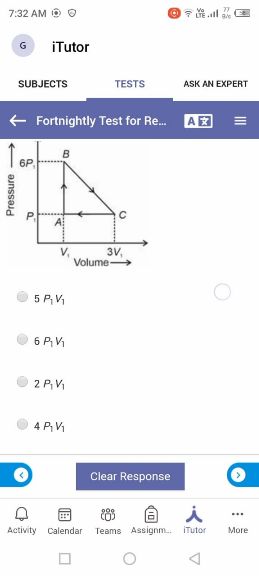
NEET neet - Physics
Asked by takshitashu46 | 09 Feb, 2022, 06:01: PM
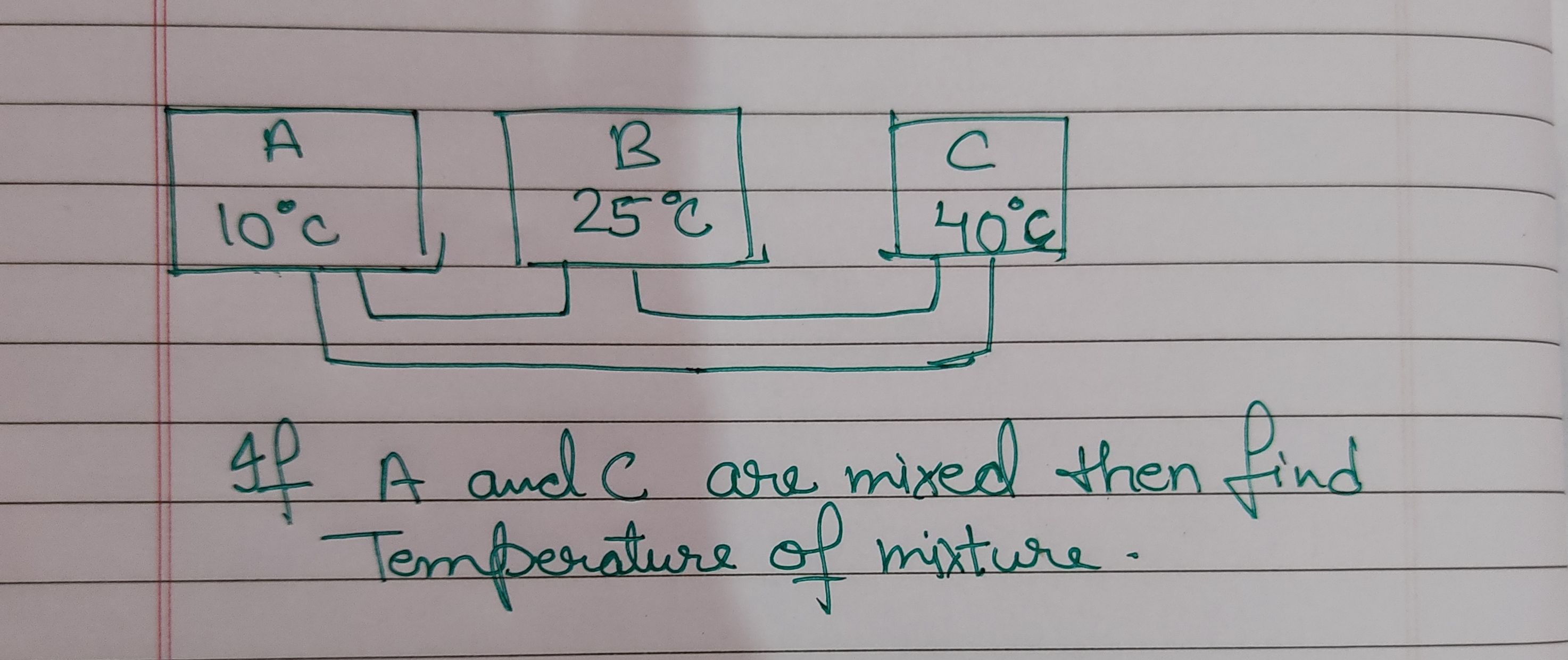
NEET neet - Physics
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 05:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 05:23: PM