CBSE Class 11-science Answered
Why mass increases with increase in temperature ? Explian in detail please
Asked by govtsecschoolnayaganv051 | 14 Dec, 2018, 07:19: PM
When the temperatue increases the volume of matter increases and density decreases. It is because the mass of that object remains same. For example, if the temperature of iron bob is increases its volume increases and density decreases while the mass is same. This is because it undergoes thermal expansion. When the temperature decreases then the volume decreases and the density increases keeping mass constant this is called contraction of substance due to the loss of heat.
Answered by Shiwani Sawant | 20 Dec, 2018, 12:41: PM
Concept Videos
CBSE 11-science - Physics
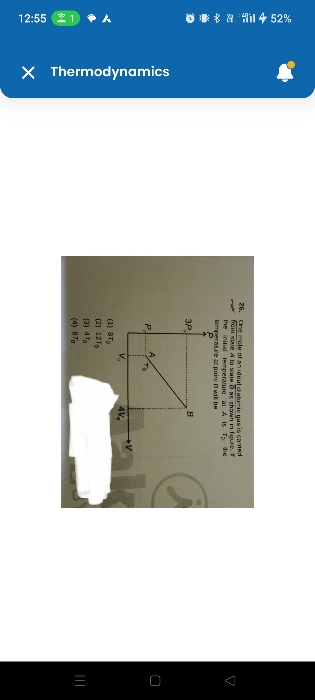
Asked by shubham23302007 | 23 Jan, 2024, 10:24: PM
CBSE 11-science - Physics
Asked by s3043632 | 22 Jan, 2023, 06:45: PM
CBSE 11-science - Physics
Asked by govtsecschoolnayaganv051 | 14 Dec, 2018, 07:19: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 10:09: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 10:11: AM
CBSE 11-science - Physics
Asked by araima2001 | 17 Mar, 2017, 08:12: AM
CBSE 11-science - Physics
Asked by Kusum and Sanjeet | 13 Jul, 2015, 10:02: PM