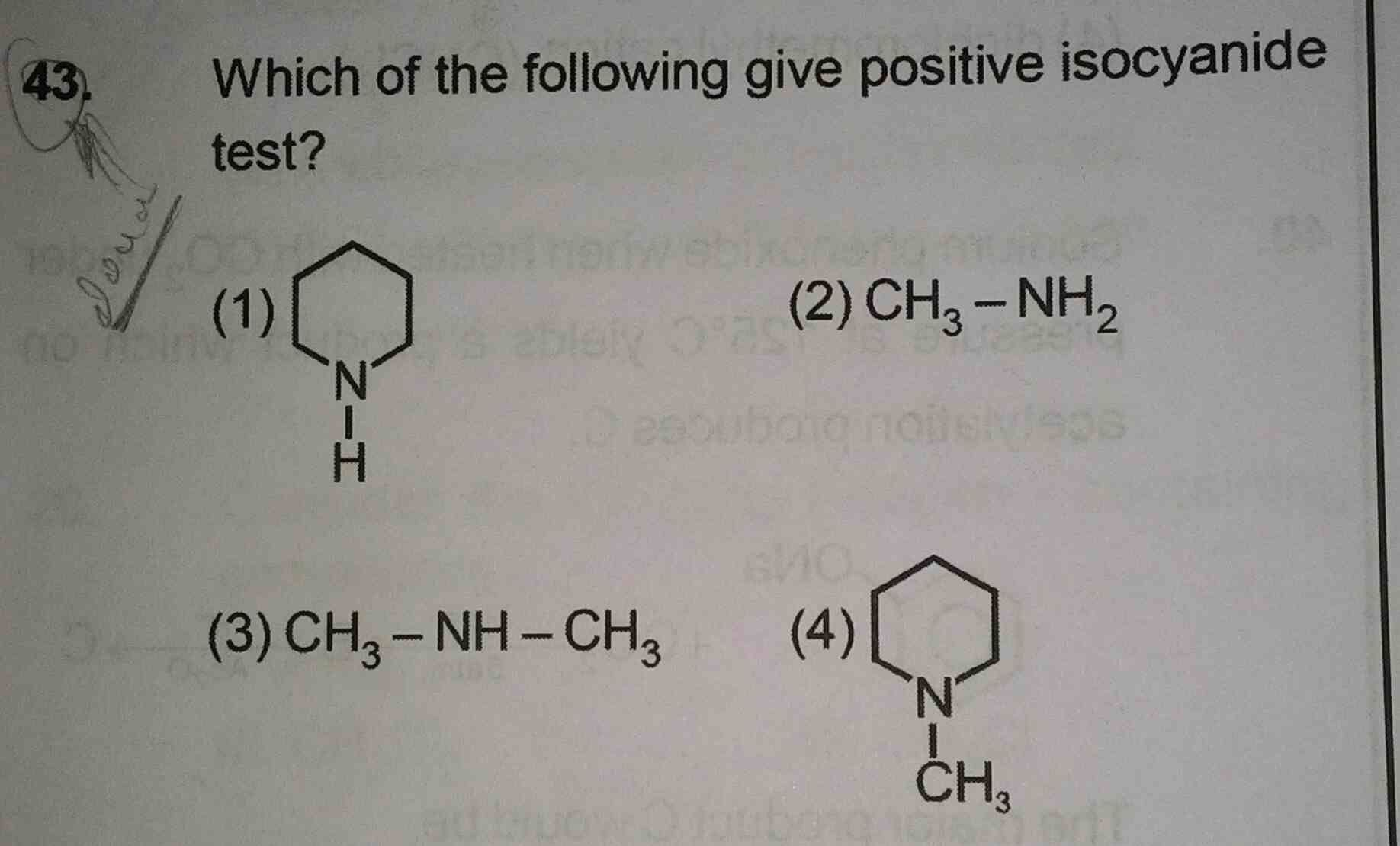
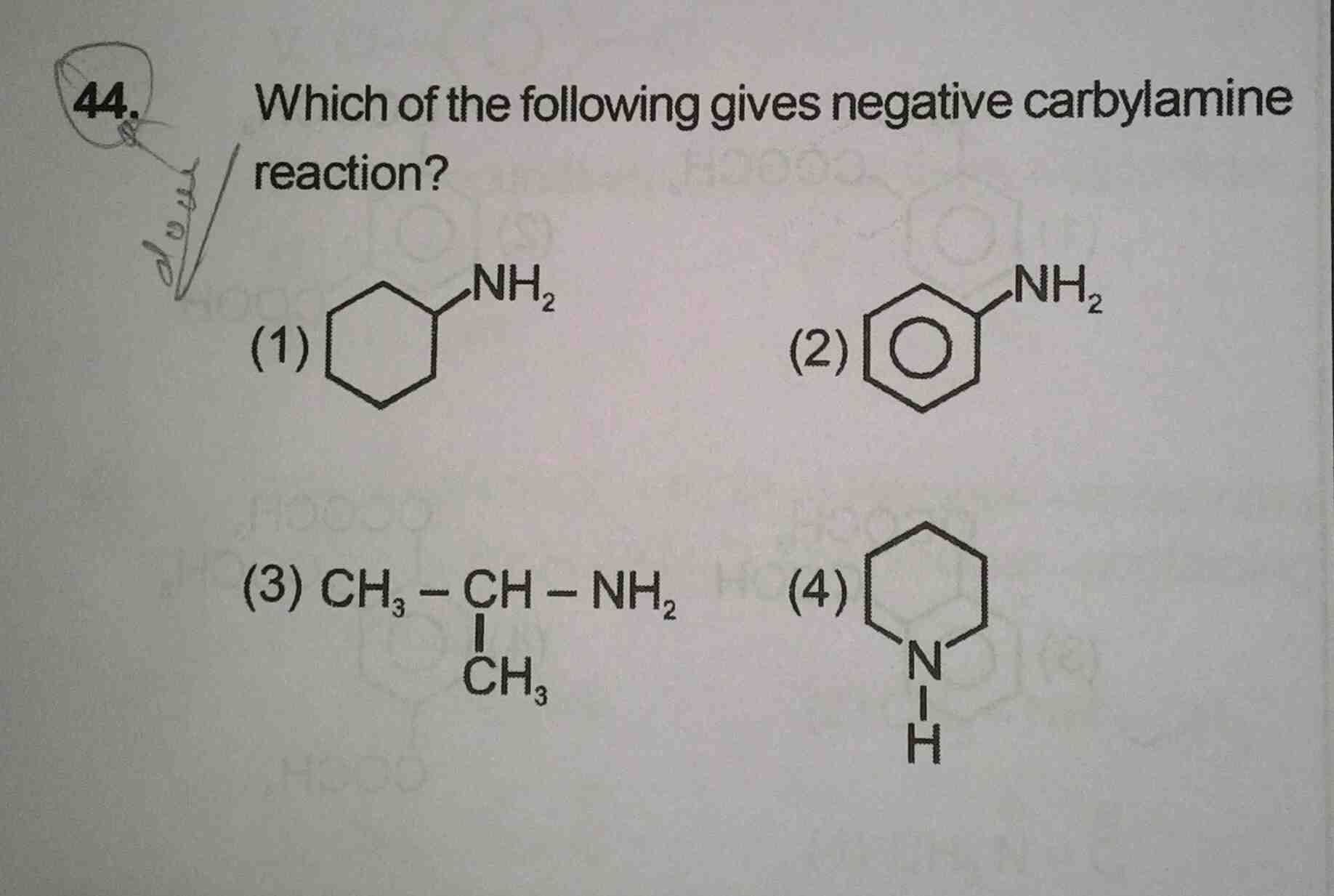
NEET Class neet Answered
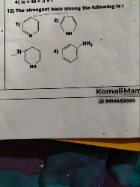
Why m-phenylenediamine is more basic than aniline? At m position NH2 exert -I effect which will reduce electron density at N then why?please explain
Asked by nipunverma59 | 19 Jan, 2019, 06:21: PM
According to Lewis theory, bases are the electron donating species.
In aniline, there is only one amino group whereas in m-phenylenediamine there are two amine groups for donation.
Hence m-phenylenediamine is more basic than aniline.
Answered by Ramandeep | 22 Jan, 2019, 11:24: AM
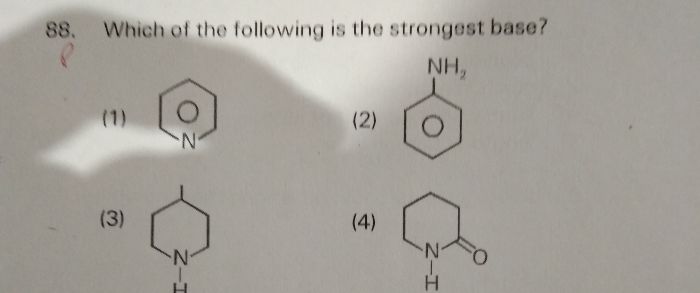
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
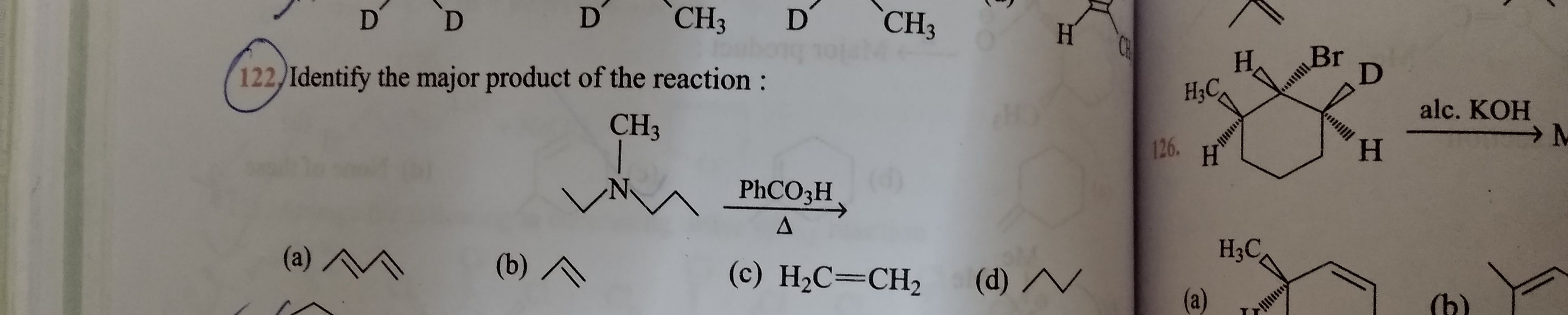
NEET neet - Chemistry
Asked by murtazan92 | 20 Nov, 2023, 11:45: PM
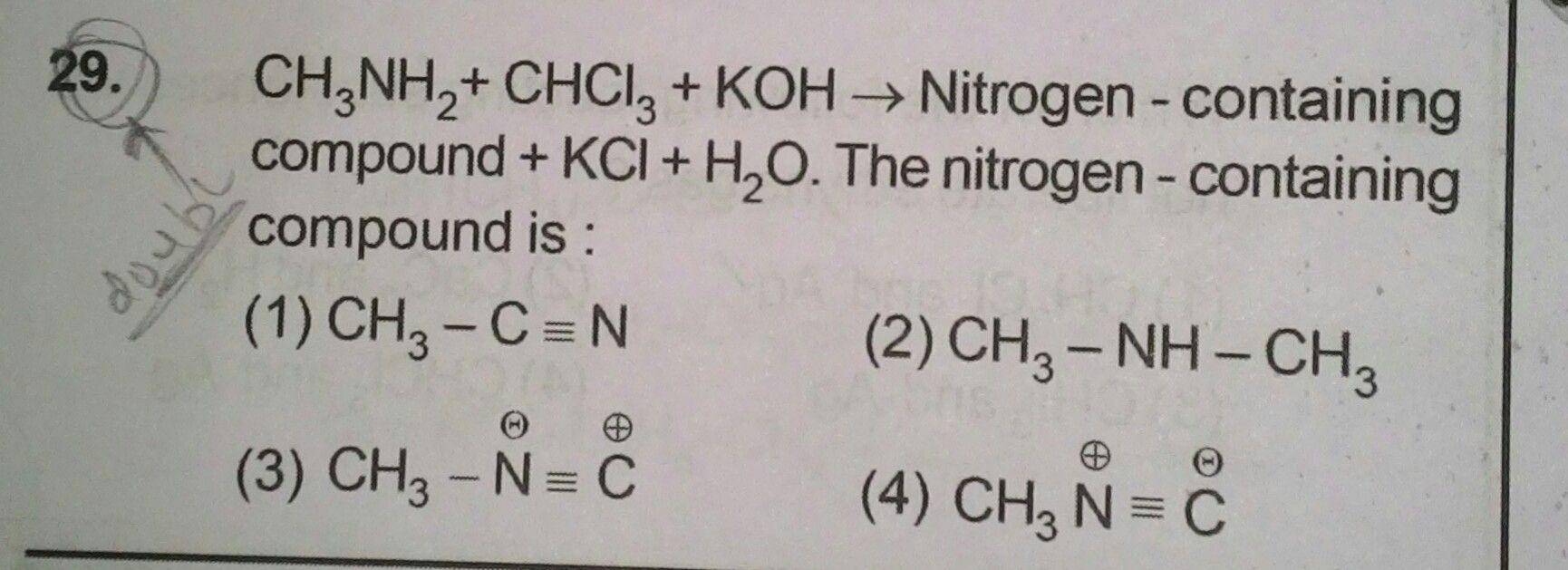
NEET neet - Chemistry
Asked by nagendrakakumuri | 19 Nov, 2023, 10:53: AM
NEET neet - Chemistry
Asked by vanshkamboj598 | 17 Aug, 2022, 02:37: AM
NEET neet - Chemistry
Asked by rohitraman1115 | 16 Feb, 2021, 08:16: PM
NEET neet - Chemistry
Asked by nameerasiddiquee90 | 05 Feb, 2021, 04:19: PM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 17 Jul, 2020, 11:12: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:50: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:44: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 26 Feb, 2020, 10:04: PM