CBSE Class 11-science Answered
why is benzene inert towards most the oxidicing agents?also mention the nitrating mixture used to nitrate benzene.
Asked by ruchira121996 | 27 Mar, 2013, 09:50: AM
In benzene, the p orbitals of the carbon atoms above and below the plane of the molecule overlap sideways with each other. This means that 6 pi electrons are found in a delocalised electron cloud above and below the plane of the molecule. This is represented by a ring in the displayed formula.
However, because the electrons are spread out across the molecule, the overall electron density is less than that of a conventional double bond.
However, because the electrons are spread out across the molecule, the overall electron density is less than that of a conventional double bond.
Oxidising agents, like KMnO4 reacts with localized pi bonds as in alkenes and alkynes, in benzene there are delocalized pi bonds (pi electronic cloud) so KMnO4 can not react.
The nitrating mixture is H2SO4 + HNO3
Answered by | 31 Mar, 2013, 06:41: AM
Concept Videos
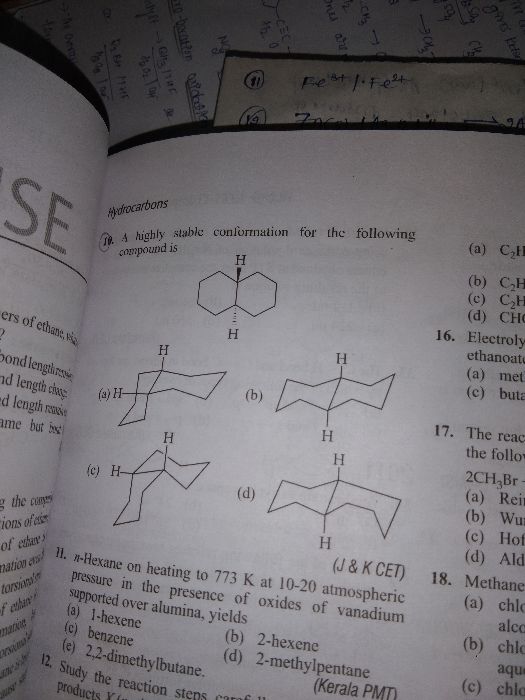
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM