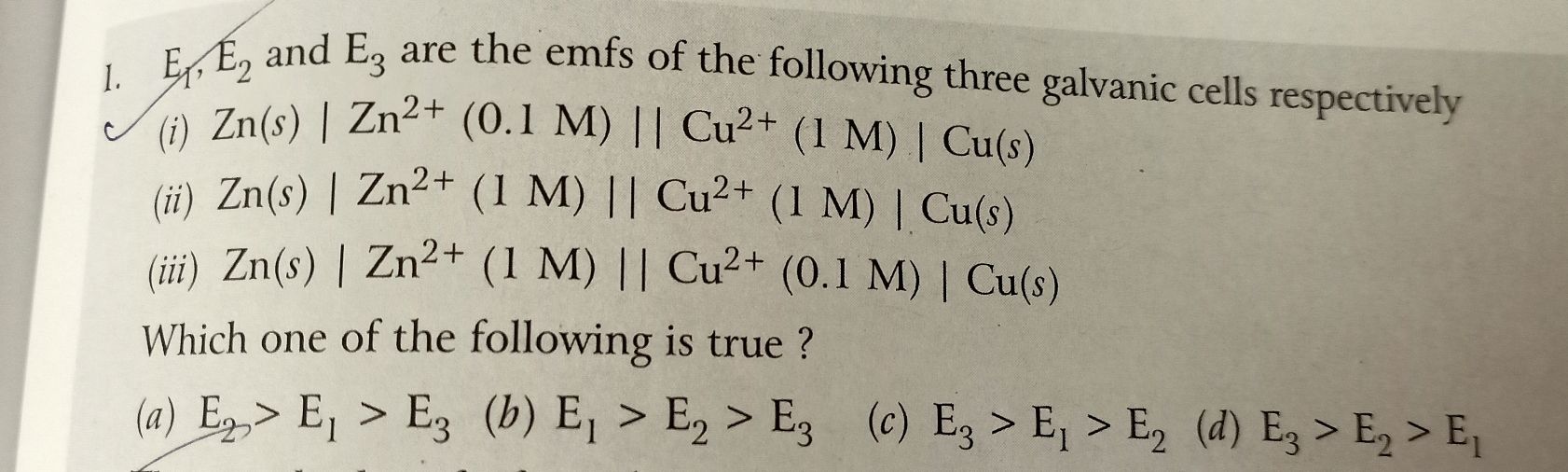CBSE Class 12-science Answered
why in this question Fe is taken as anode and zinc as cathode whereas according to electrochemical series Fe lies below zinc??? Also mention the soln for the above ques

Asked by ayushi | 07 Jul, 2016, 04:25: PM
According to given reaction, anode is zinc where oxidation takes place and cathode is Fe wher reduction takes place.
We have to reverse both half cell reactions as oxidation potentials are given.
Hence,
Zn2+ (aq) + 2e- → Zn(s) , Eº = -0.76 V
Fe2+(aq) + 2e- → Fe(s) , Eº = -0.44 V
Half cell reaction with more positive reduction potential will reverse the half cell reaction with less positive reduction potential.
Hence emf of the cell is,
Eº (RHS) - Eº (LHS) = Eº(cell)
-0.76 V - (- 0.44 V) = 0.32 V
Thus, option (b) is the correct option.
Answered by Prachi Sawant | 08 Jul, 2016, 03:43: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rajpatil | 19 Jun, 2020, 07:12: PM
CBSE 12-science - Chemistry
Asked by piyushkhariyal | 06 Mar, 2020, 10:36: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 15 Feb, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 11 Nov, 2019, 12:09: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Nov, 2019, 04:50: PM
CBSE 12-science - Chemistry
Asked by Balbir | 21 Aug, 2019, 09:14: PM
CBSE 12-science - Chemistry
Asked by ankitathapliyal097 | 24 Jul, 2019, 10:05: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 26 Jun, 2019, 02:18: PM
CBSE 12-science - Chemistry
Asked by jhajuhi19 | 01 May, 2019, 09:03: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 26 Sep, 2018, 09:00: PM