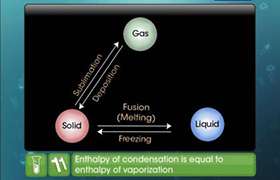
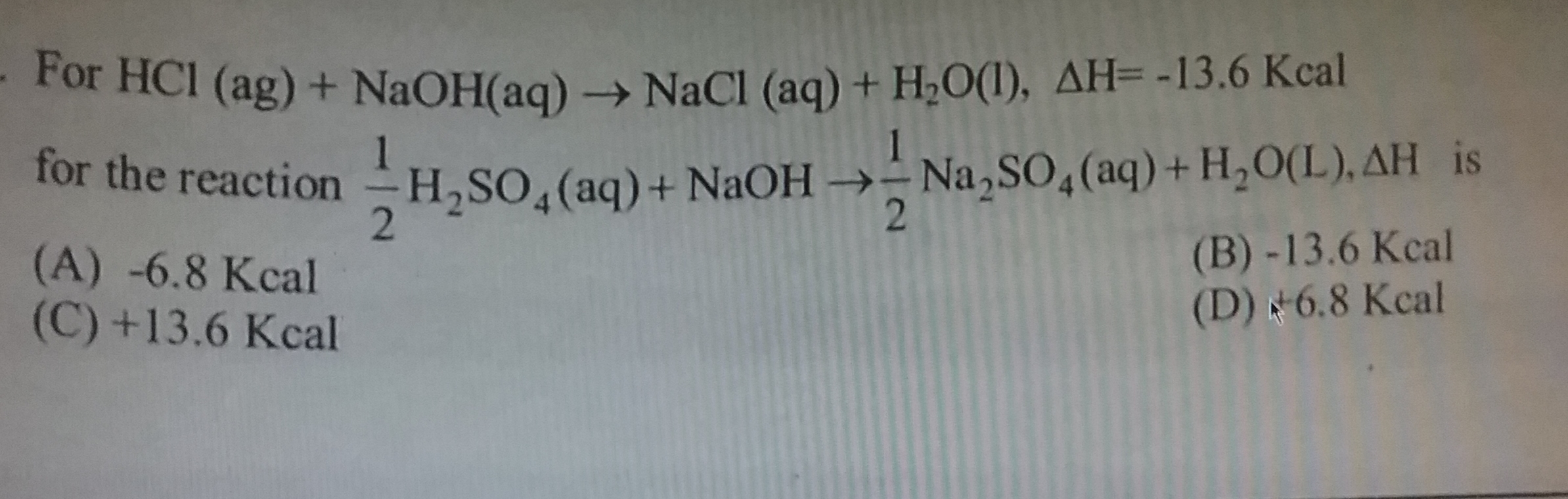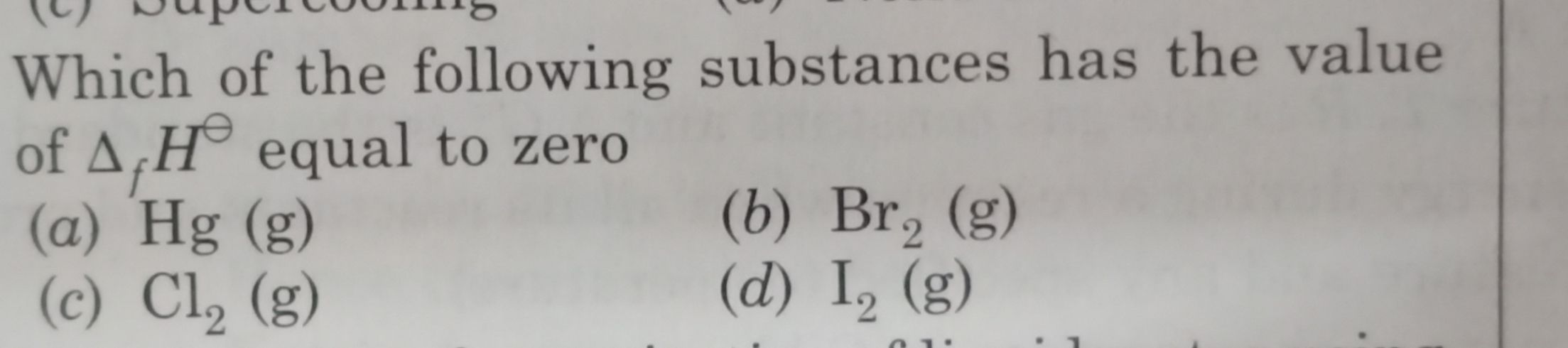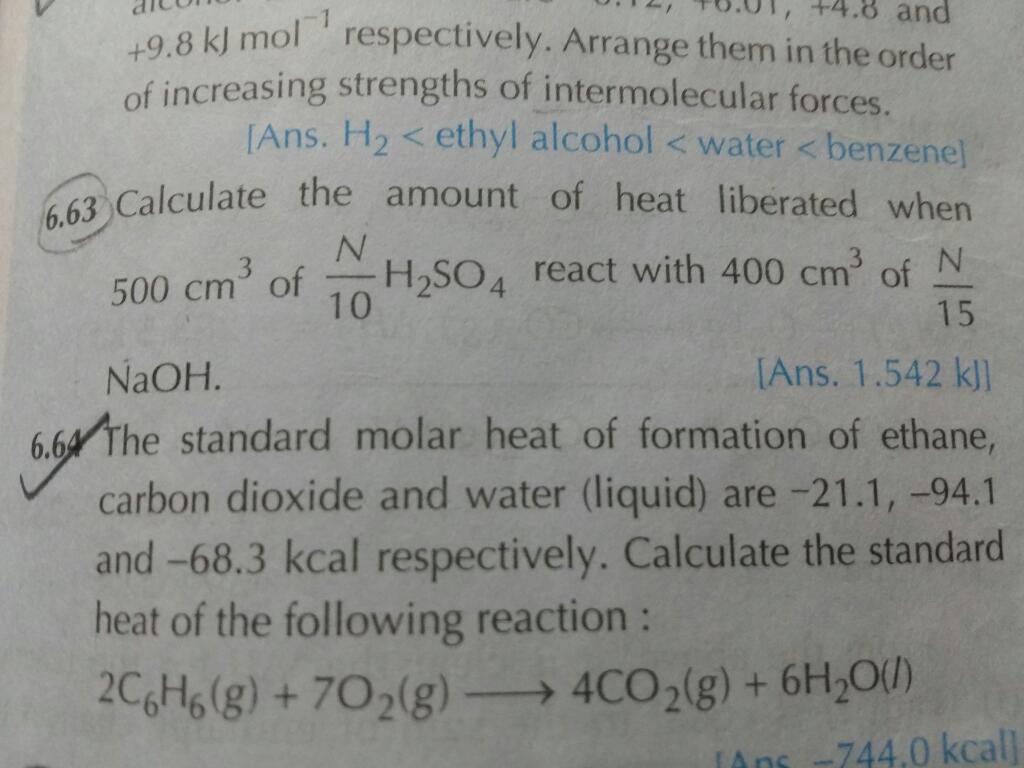CBSE Class 11-science Answered
In water molecule, two hydrogen bonds are covalently bonded to each other. Also, two molecules of water are also bonded to each other by weak hydrogen bonding. It happens when hydrogen atoms of one water molecule sits close to oxygen atom of another water molecule. Covalent bonds get stretched and store energy due to natural repulsion when two water molecules come closer. On heating, hydrogen bonds get stretched and water get less dense. This extra stretch in hydrogen bonding relax covalent bonds which releases stored energy. This process of covalent giving up energy is equivalent to cooling. Hence warm water freezes faster than normal cold water.
This effect is called Mpemba effect.