CBSE Class 11-science Answered
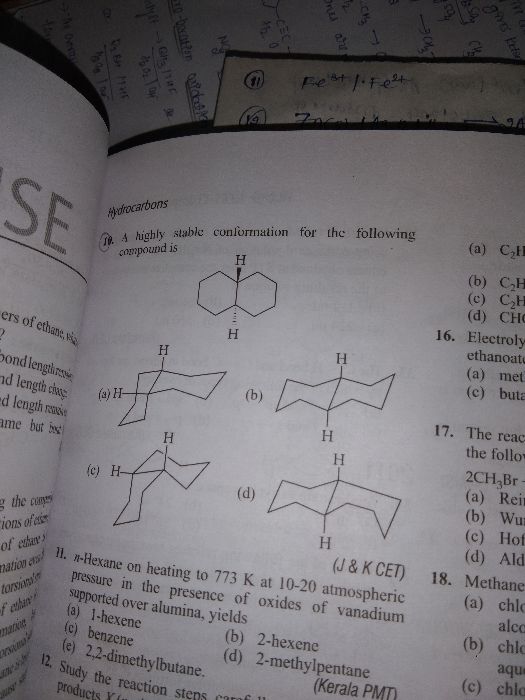
why does conformation arise in alkanes,while some alkenes undergo geomertical isomerism ?
Asked by ruchira121996 | 26 Mar, 2013, 07:33: PM
Geometrical isomerism is possible for those compounds in which free rotation about bond is not possible in alkenes a double bond restrict the free rotation so arrangement of substituents on double bounded carbon may occur in two ways, same groups at same side of the plane of double bond (Cis) or at opposite side (Trans).
Answered by | 27 Mar, 2013, 06:40: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM