CBSE Class 7 Answered
Yes there is a reaction which takes place.
When the indicator reacts with H+ ions of acid it forms a species which has some colour or is coloureless. When the indicator reacts with OH- of bases it forms some other species which has a different colour.
An indicator is a large organic molecule that works somewhat like a " color dye". Whereas most dyes do not change color with the amount of acid or base present, there are many molecules, known as acid - base indicators , which do respond to a change in the hydrogen ion concentration. Most of the indicators are themselves weak acids.
The most common indicator is found on "litmus" paper. It is red below pH 4.5 and blue above pH 8.2.
Color
Blue Litmus
Red Litmus
Acid
turns red
stays same
Base
stays same
turns blue
Other commercial pH papers are able to give colors for every main pH unit. Universal Indicator, which is a solution of a mixture of indicators is able to also provide a full range of colors for the pH scale.
A variety of indicators change color at various pH levels. A properly selected acid-base indicator can be used to visually "indicate" the approximate pH of a sample. An indicator is usually some weak organic acid or base dye that changes colors at definite pH values. The weak acid form (HIn) will have one color and the weak acid negative ion (In-) will have a different color. The weak acid equilibrium is:
HIn --> H+ + In-
For phenolphthalein: pH 8.2 = colorless; pH 10 = red
For bromophenol blue: pH 3 = yellow; pH 4.6 = blue
Litmus
Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to HLit. The "H" is the proton which can be given away to something else. The "Lit" is the rest of the weak acid molecule.
There will be an equilibrium established when this acid dissolves in water. Taking the simplified version of this equilibrium:

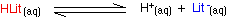
The un-ionised litmus is red, whereas the ion is blue.
Now use Le Chatelier's Principle to work out what would happen if you added hydroxide ions or some more hydrogen ions to this equilibrium.
The most common indicator is found on "litmus" paper. It is red below pH 4.5 and blue above pH 8.2.
| Color | Blue Litmus | Red Litmus |
| Acid | turns red | stays same |
| Base | stays same | turns blue |
Other commercial pH papers are able to give colors for every main pH unit. Universal Indicator, which is a solution of a mixture of indicators is able to also provide a full range of colors for the pH scale.
A variety of indicators change color at various pH levels. A properly selected acid-base indicator can be used to visually "indicate" the approximate pH of a sample. An indicator is usually some weak organic acid or base dye that changes colors at definite pH values. The weak acid form (HIn) will have one color and the weak acid negative ion (In-) will have a different color. The weak acid equilibrium is:
HIn --> H+ + In-
For phenolphthalein: pH 8.2 = colorless; pH 10 = red
For bromophenol blue: pH 3 = yellow; pH 4.6 = blue
Litmus
Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to HLit. The "H" is the proton which can be given away to something else. The "Lit" is the rest of the weak acid molecule.
There will be an equilibrium established when this acid dissolves in water. Taking the simplified version of this equilibrium:
![]()
![]()
The un-ionised litmus is red, whereas the ion is blue.
Now use Le Chatelier's Principle to work out what would happen if you added hydroxide ions or some more hydrogen ions to this equilibrium.

