CBSE Class 11-science Answered
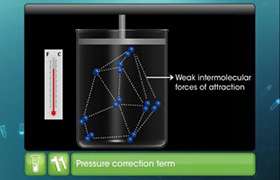
Why do gases deviate from ideal behaviour?
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM
The behavior of real gases usually agrees with the predictions of the ideal gas equation to within 5% at normal temperatures and pressures. At low temperatures or high pressures, real gases deviate significantly from ideal gas behavior.
Real gases deviate from ideal gas law because their molecules interact with each other. At high pressure the molecules of gases are very close to each other so the molecular interactions start operating and these molecules do not strike the walls of the container with full impact. Thus the pressure exerted by the gas is lower than the pressure exerted by the ideal gas. At high pressure the repulsive forces also come into action .So the constants of pressure and volume are corrected and the equation is written as:
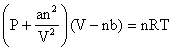
This equation is known as van der Waals equation.
Answered by | 17 Jun, 2016, 02:01: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM