NEET Class neet Answered
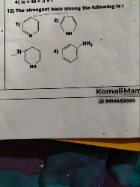
Why Aniline is more basic than Pyrole?
Asked by rohitraman1115 | 16 Feb, 2021, 08:16: PM

In Pyrol, the lone pair of electron of N is delocalized because it is an aromatic compound. Hence lone pair is the part of the sextet. this means that these electrons are very stable right where they are ( in the aromatic system), and are less available for bonding to proton for these reasons pyrrole nitrogens are not strongly basic

On other hands, aniline is also aromatic but the lone pair of electrons of NH2 group in aniline is delocalized over the benzene ring, it is not involved in aromatization. This lone pair is still available for proton, hence Aniline is the stronger base than Pyrrole.

Answered by Ramandeep | 16 Feb, 2021, 10:21: PM
NEET neet - Chemistry
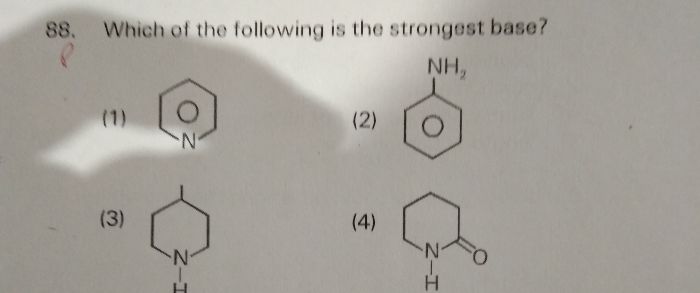
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
NEET neet - Chemistry
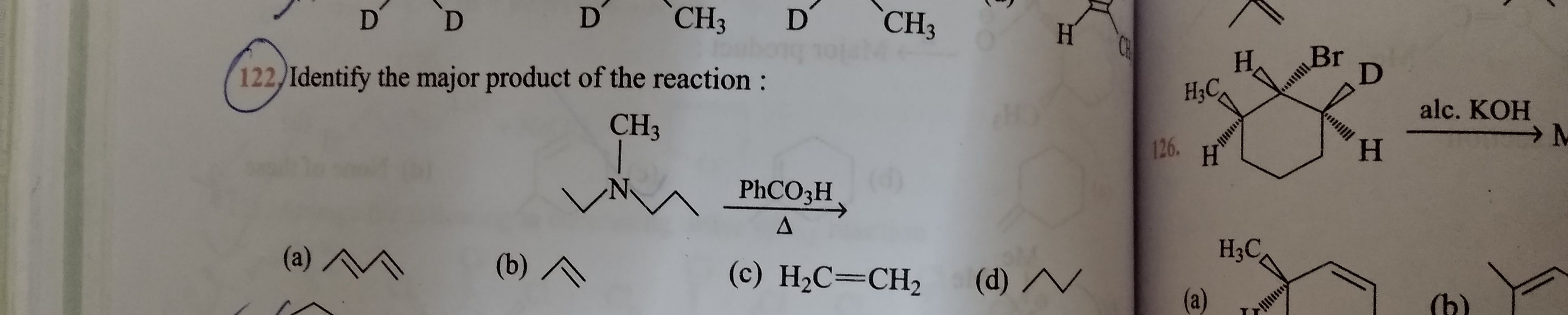
Asked by murtazan92 | 20 Nov, 2023, 11:45: PM
NEET neet - Chemistry
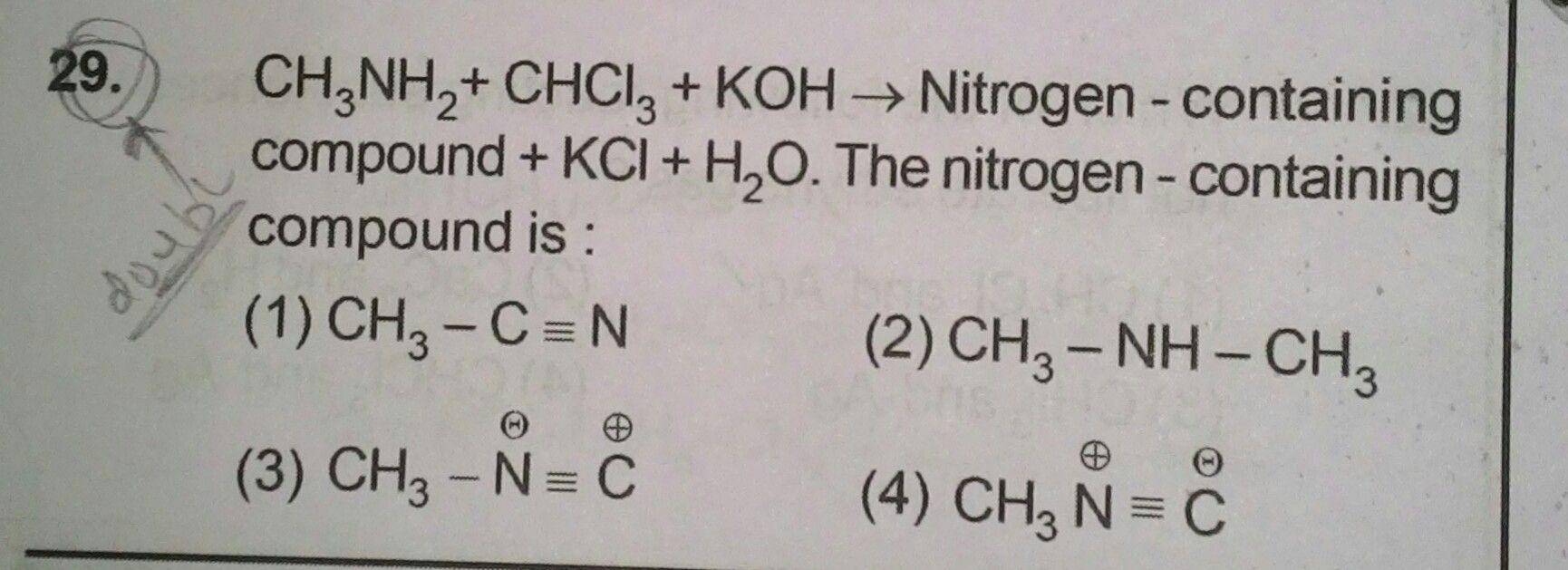
Asked by nagendrakakumuri | 19 Nov, 2023, 10:53: AM
NEET neet - Chemistry
Asked by vanshkamboj598 | 17 Aug, 2022, 02:37: AM
NEET neet - Chemistry
Asked by rohitraman1115 | 16 Feb, 2021, 08:16: PM
NEET neet - Chemistry
Asked by nameerasiddiquee90 | 05 Feb, 2021, 04:19: PM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 17 Jul, 2020, 11:12: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:50: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:44: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 26 Feb, 2020, 10:04: PM