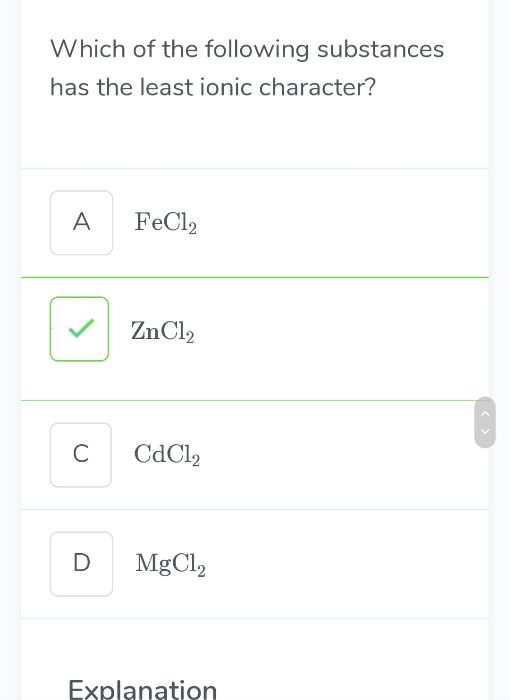
CBSE Class 11-science Answered
which of the following two compounds would you expect to have a higher lattice energy value-KCL or NaCl?
Asked by rajenderdagar123 | 24 Dec, 2016, 09:42: PM
Lattice energy depends on the magnitude of the charges of the ions and the distance between them.
Comparing NaCl and KCl, we see that the lattice energies are: 788, 699 kJ/mol, respectively.
Since the anion is the same for all three salts and the cations are all +1, the only difference between these has to be in the distance between the ions.
Answered by Vaibhav Chavan | 24 Dec, 2016, 09:52: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:34: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 28 Sep, 2019, 03:17: AM
CBSE 11-science - Chemistry
Asked by ashutosharnold1998 | 25 Sep, 2019, 02:20: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 07 Oct, 2014, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Jun, 2016, 10:52: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 29 Jul, 2015, 11:11: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 07 Oct, 2014, 04:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 07 Oct, 2014, 04:49: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 07 Oct, 2014, 04:54: PM