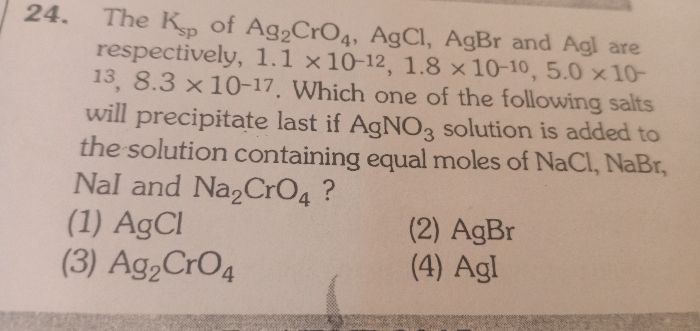CBSE Class 11-science Answered
Which is the correct increasing order of the solubility of silver chloride in 0.1 M solution of the above compounds.
(a) Sodium chloride
(b)sodium nitrate
(c)silver chromate
Options
(1)a

Asked by jhajuhi19 | 12 Jun, 2019, 07:23: PM
Solubility is reduced due to common ion effect.
When AgCl is dissolved it releases Ag+ and Cl- ions
(a) NaCl -NaCl will release Na+ and Cl- .

So due to increase in concentration of common ion ( Cl-) rection will move in backward direction and solubility decreased.
(b)

There is no common ion effect so solubility of AgCl will not decreased.
(c)

Here common ion effect is due to Ag+ ions. So solubility is decreased.
This decrease in solubility will be more as compared to NaCl because in NaCl, 1 Cl- ion is available for one AgCl molecule, In Ag2CrO4, 2 Ag+ ions are available for one AgCl molecule. So common ion effect in Ag2CrO4 is more effective.
Order of solubility -
b>a>c
Answered by Ravi | 24 Jun, 2019, 03:23: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 02:33: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 02:32: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 18 Apr, 2020, 02:21: PM
CBSE 11-science - Chemistry
Asked by Anish | 23 Aug, 2019, 01:48: AM
CBSE 11-science - Chemistry
Asked by jhajuhi19 | 12 Jun, 2019, 07:23: PM
CBSE 11-science - Chemistry
Asked by Prakash | 28 Jun, 2018, 06:09: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 06:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 May, 2015, 03:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 02:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:24: PM