NEET Class neet Answered
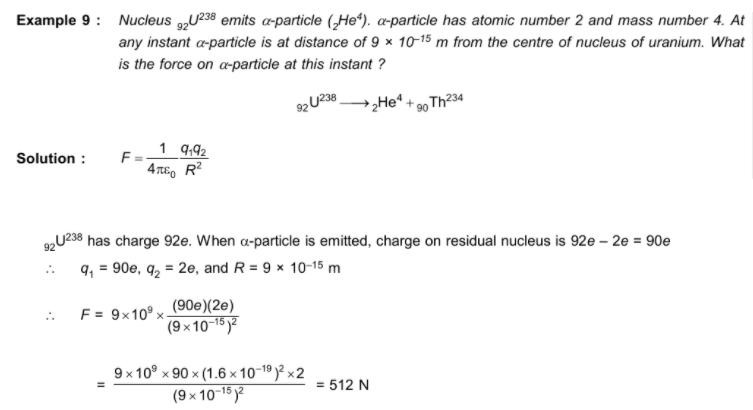
When we calculate coulomb's force between the Th atom and the alpha particle, why do we not consider the attractive forces on the alpha particle due to the electrons of the Th atom?
Asked by akdwadasi1111 | 20 Apr, 2021, 12:19: PM
This is due to size of atom and size of nucleus. Size of atom is around 10-12 m .
Nucleus is concentrated at centre of atom and its size is 10-15 m .
Hence when α particle is at a distance 9 × 10-15 m from centre of nucleus/atom ,
it is very close to +ve charge nucleus . Hence Coulombic repulsive foce between nucleus and α particle
is much greater than attaractive force due to electron
Answered by Thiyagarajan K | 20 Apr, 2021, 02:49: PM
Concept Videos
NEET neet - Physics
Asked by roshanrocky334 | 13 Jan, 2024, 11:52: AM
NEET neet - Physics
Asked by adititiwari601 | 13 Jun, 2022, 07:44: AM
NEET neet - Physics
Asked by begfatima123 | 06 May, 2022, 11:37: PM
NEET neet - Physics
Asked by jhajuhi19 | 30 Aug, 2021, 08:02: PM
NEET neet - Physics
Asked by akshadevdm2020 | 22 May, 2021, 03:43: PM
NEET neet - Physics
Asked by akdwadasi1111 | 20 Apr, 2021, 12:19: PM
NEET neet - Physics
Asked by Prashant DIGHE | 10 Apr, 2020, 09:21: PM