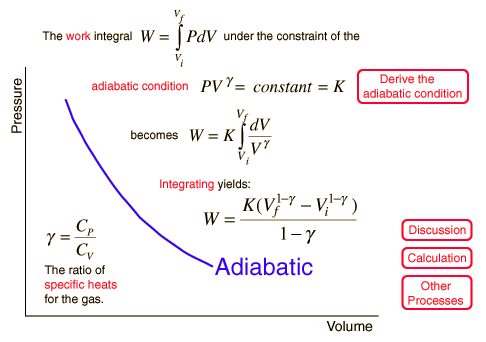
An adiabatic process is one in which no heat is gained or lost by the system. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time, so that there is no opportunity for significant heat exchange.
The specific heat of a gas is the heat energy added to the gas per degree of temperature rise.The value of the specific heat depends on the condition under which the heat is added. If the heatis added at constant volume the heat energy goes entirely into increasing the internal kinetic energyof the particles. If the heat is added at constant pressure, the gas can expand while the heat isadded and the gas does work on the expanding walls of the container. Consequently, the specificheat at constant pressure (Cp) is greater than the specific heat at constant volume (Cv). the two are related byCp = Cv + R . By applying the first law of thermodynamics and using the adiabatic condition of zero heattransfer, it can be shown that the pressure and volume of an ideal gas obey the equation (called the adiabatic law)pVg = constant,during an adiabatic process. The exponent g is the ratio of the specific heats, Cp / Cv .