CBSE Class 11-science Answered
what is the diference between entropy and enthalpy?
what is the gibbs energy?
Asked by svmic.dbr | 09 Jan, 2015, 10:33: PM
- Entropy is basically a term for disorder or randomness while enthalpy is defined as the total heat content of a system at constant pressure equivalent to sum of internal energy and pressure-volume energy is called the enthalpy of the system and is given by the following equation
H = U + PV,
Here U is the internal energy.
- S.I. unit of Entropy is Joules per Kelvin while S. I. unit of enthalpy is Joule per kilogram.
The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system.
G = H - TS
Answered by Arvind Diwale | 11 Jan, 2015, 08:53: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by mankdubey670 | 06 Jun, 2022, 01:27: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 06:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 04:44: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:46: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 04:51: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 04:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 05:00: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:46: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Sep, 2014, 03:28: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Sep, 2014, 03:39: PM




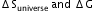 ?
?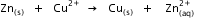 Given that
Given that 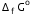 for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.
for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.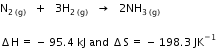 Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.
Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.