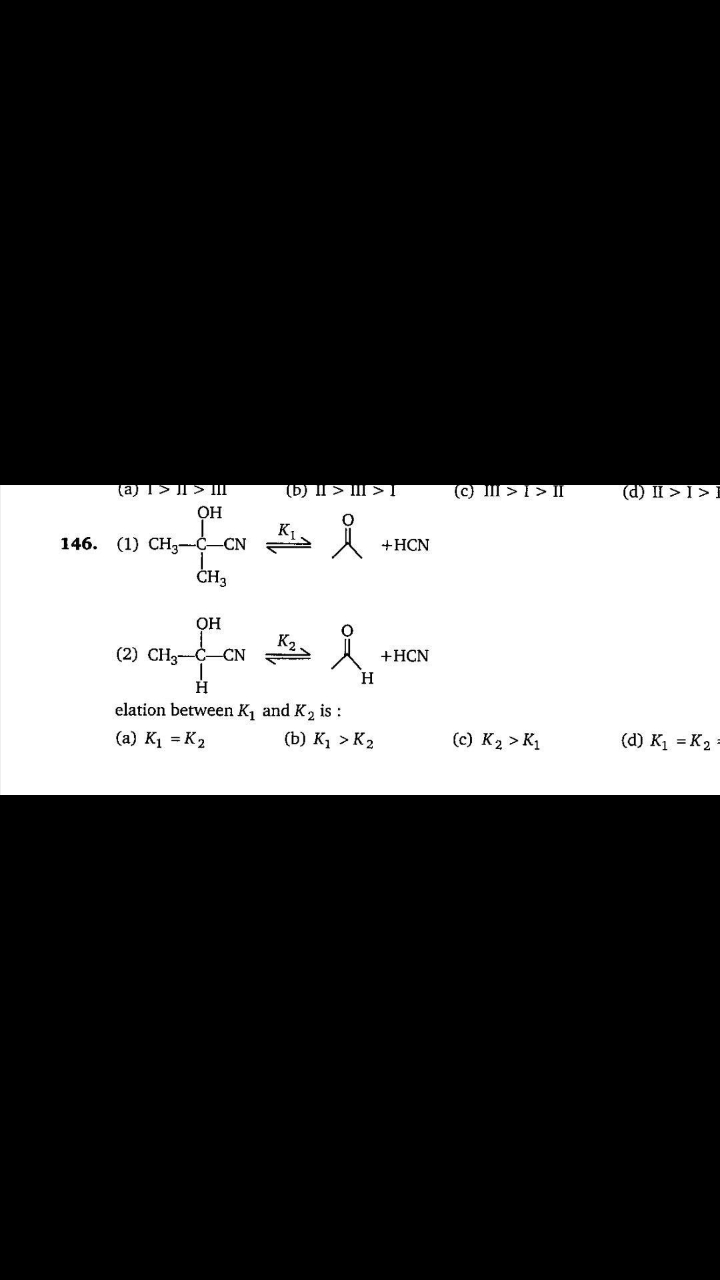
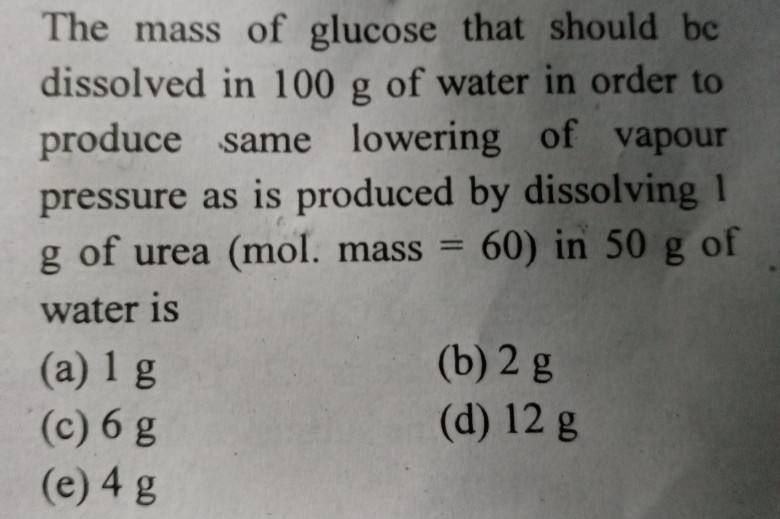NEET Class neet Answered
What is the composition of the vapour which is in equilibrium at 30 degree celcius with a benzene-toluene solution with a mole fraction of benzene of 0.400? (P0 B is 119 torr and P0 T is 37 torr) (a) 1.237 (b) 2.237 (c) 3.237 (d) 0.237
Asked by Balbir | 28 Jul, 2019, 07:59: PM
Q. What is the composition of the toluene vapour which is in equilibrium at 30 degree celcius with a benzene-toluene solution with a mole fraction of benzene of 0.400? (P0 B is 119 torr and P0 T is 37 torr)
Given:
T = 30 °C
PB0 = 119 torr
PT0 = 37 torr
PT = XT PT0
Mole fraction of benzene = 0.4
Mole fraction of ttoluene = 1 - 0.4
= 0.6
According to Raoult's law,
PB = XBP0
PT = XT PT0
Total pressue = PB + PT ---(1)
By putting values in eq (1)
PTot = XBPB0 + XT PT0
= (0.4 × 119) + (0.6×37)
= 69.8 torr
Mole fraction of toluene in vapour phase
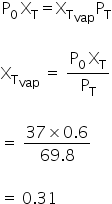
Answered by Varsha | 28 Jul, 2019, 11:57: PM
Concept Videos
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 17 May, 2020, 03:07: PM