CBSE Class 10 Answered
What is significance of reaction of bromine in with alkenes and alkynes?
Asked by naveen.mohandas | 12 Feb, 2011, 09:09: PM
Dear Student
Unsaturated hydrocarbons such as alkenes and alkynes are much more reactive than the parent alkanes. They react rapidly with bromine, for example, to add a Br2 molecule across the C=C double bond.
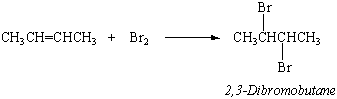
This reaction provides a way to test for alkenes or alkynes. Solutions of bromine in CCl4 have an intense red-orange color. When Br2 in CCl4 is mixed with a sample of an alkane, no change is initially observed. When it is mixed with an alkene or alkyne, the color of Br2 rapidly disappears.
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 13 Feb, 2011, 10:19: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by agarwalkrishnam98 | 01 Oct, 2023, 08:28: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:01: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM











