what is ruther ford model of an atom?
Rutherford's Atomic Model:
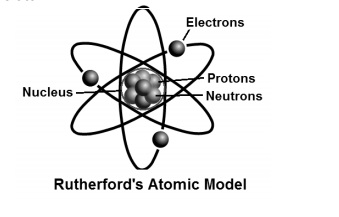
Based on the results of the α-particle scattering experiments, Rutherford put forth his atomic model.
An atom contains a positively charged centre called the nucleus of the atom. Almost all the mass of
the atom is concentrated in the nucleus.
The electrons of the atom revolve around the nucleus in fixed, circular orbits.
The size of the nucleus is many times smaller than the size of the atom. The nucleus of an atom is
10,000 times smaller than the atom.
Drawbacks of Rutherford’s Model of an Atom
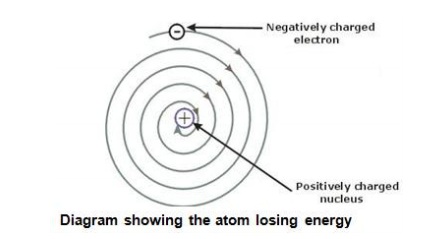
Rutherford’s atomic model could not explain how moving electrons could remain in their orbits.
Any charged particle during acceleration would radiate energy, and while revolving, it would lose its
energy and eventually fall into the nucleus.
This means that the atom would be highly unstable.
But, matter is composed of stable atoms.
Thus, the major drawback of Rutherford’s atomic model was that it could not explain the stability
of atoms.
Rutherford’s Model of an atom is similar to that of the Solar system.
Just as in the solar system, the Sun is at the centre and the planets
revolve around it, in an atom, the electrons revolve around the centrally
located nucleus containing protons.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Classification of Elements and Periodicity in Properties
- 2 Chemical Bonding and Molecular Structure
- 3 States of Matter
- 4 Equilibrium
- 5 Hydrogen
- 6 Hydrocarbons
- 7 Environmental Chemistry
- 8 Solutions
- 9 Chemical Kinetics
- 10 Surface Chemistry
- 11 Biomolecules
- 12 Polymers
- 13 Chemistry in Everyday Life
- 14 Atomic Structure
- 15 Chemical Thermodynamics
- 16 Redox Reactions and Electrochemistry
- 17 p-Block Elements
- 18 d - and f - Block Elements
- 19 Some Basic Principles of Organic Chemistry
- 20 Organic Compounds Containing Halogens
- 21 Organic Compounds Containing Oxygen
- 22 Organic Compounds Containing Nitrogen
- 23 Co-ordination Compounds
- 24 Purification and Characterisation of Organic Compounds
- 25 s-Block Element (Alkali and Alkaline Earth Metals)
- 26 Solid State
- 27 Some Basic Concepts in Chemistry
- 28 General Principles and Processes of Isolation of Metals
- 29 Principles Related to Practical Chemistry
