CBSE Class 11-science Answered
What is Planck's quantum theory
Asked by Prateek Dubey | 10 Sep, 2013, 04:15: PM
Max Plank in 1901 , gave a new revolutionary theory known as Quantum theory of radiation.
Main features of this theory are :
- radiant energy is not emitted or absorbed continously but discontinously in the form of small packets of energy called Quanta. Each quanta is associated with a definite amount of energy .
- E is directly proportional to frequency of light,
3. The total amount of energy emitted or absorbed by the body will be some whole no. multiple of quantum ,i.e,
E=nhv , where n is an integer such as 1, 2, 3, ....
Since, the frequency 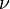 , wavelength ?, and speed of light c are related by ?? = c, the Planck relation for a photon can also be expressed as
, wavelength ?, and speed of light c are related by ?? = c, the Planck relation for a photon can also be expressed as
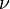 , wavelength ?, and speed of light c are related by ?? = c, the Planck relation for a photon can also be expressed as
, wavelength ?, and speed of light c are related by ?? = c, the Planck relation for a photon can also be expressed asThe above equation leads to another relationship involving the Planck constant.
Answered by Hanisha Vyas | 10 Sep, 2013, 07:17: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 03:37: PM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 02:48: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 04:15: PM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 09:14: PM
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by advssdrall | 12 Jan, 2022, 05:12: AM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 06:46: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 22 Oct, 2021, 07:29: PM










