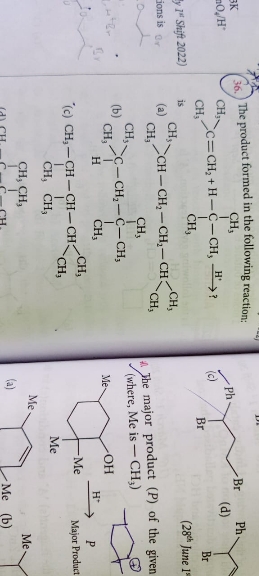
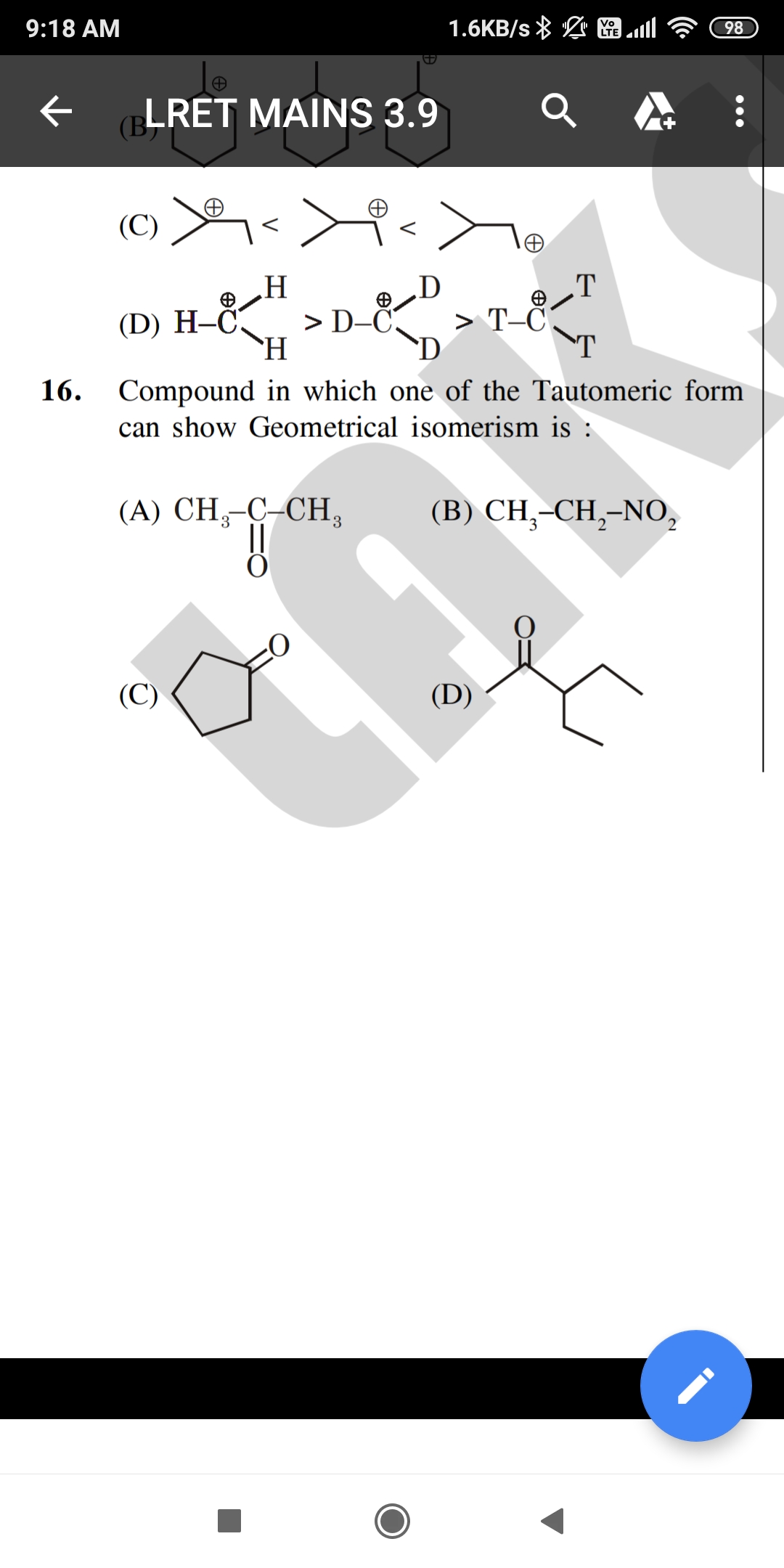
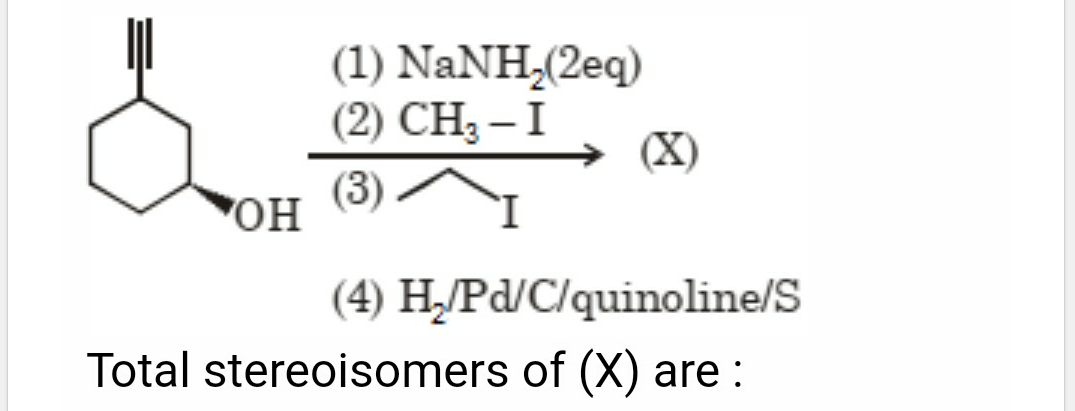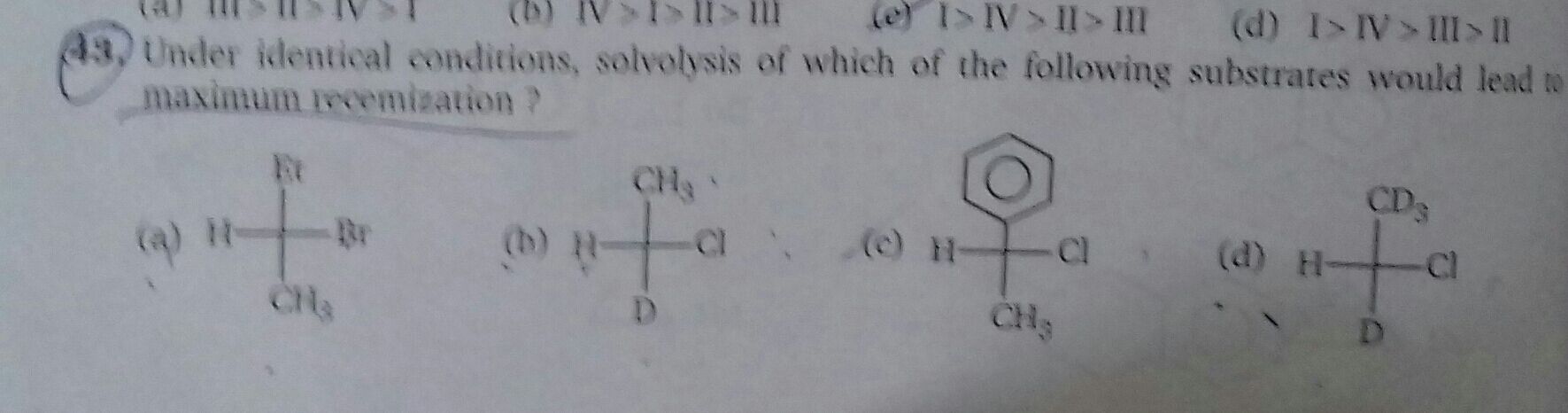JEE Class main Answered
What is more stable, unsaturated free radical or saturated free radical?
Asked by bb ki | 10 Feb, 2020, 09:33: PM
Free radical: An atom or group of atoms having an unpaired electron.
Order of stability of the species: CH3• < 1º < 2º < 3º
Reason for stability order: Larger the number of alkyl groups attached to the carbon atom carrying the odd electron, greater is the delocalization of the odd electron and hence more stable is the free radical.
The stability of a free radical decreases as the orbital is held closer to the nucleus.

Answered by Ramandeep | 12 Feb, 2020, 11:54: AM
JEE main - Chemistry
Asked by patelamrutbhaib24 | 28 Jan, 2024, 01:44: PM
JEE main - Chemistry
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
JEE main - Chemistry
Asked by mp797056 | 23 Oct, 2023, 04:23: PM
JEE main - Chemistry
Asked by dheerajrao2005 | 26 Mar, 2022, 07:06: PM
JEE main - Chemistry
Asked by yasharthshankar | 05 Jun, 2020, 11:39: PM
JEE main - Chemistry
Asked by jhajuhi19 | 28 Mar, 2020, 10:45: AM
JEE main - Chemistry
Asked by bb ki | 10 Feb, 2020, 09:33: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 29 Dec, 2019, 01:05: PM