CBSE Class 9 Answered
What is MOLAR MASS?? Write around 7-8 lines on it..
Asked by | 24 Jul, 2012, 02:02: PM
Molar mass 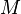 is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. It is a physical property of a given substance. The base SI unit for molar mass is kg/mol.
is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. It is a physical property of a given substance. The base SI unit for molar mass is kg/mol.
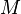 is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. It is a physical property of a given substance. The base SI unit for molar mass is kg/mol.
is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. It is a physical property of a given substance. The base SI unit for molar mass is kg/mol. One mole of hydrogen atoms has a mass of 1.008 g and 1 mol of oxygen atoms has a mass of 16.00 g, so 1 mol of water has a mass of (2 x 1.008 g) + 16.00 g = 18.02 g. The molar mass of water is 18.02 g/mol.
In general, the molar mass of any molecular compound is the mass in grams numerically equivalent to the sum of the atomic masses of the atoms in the molecular formula:
The formula mass of methane, CH4, is 12.01 + (1.008 x 4) = 16.04 u and its molar mass is 16.04 g/mol.
The formula mass of methane, CH4, is 12.01 + (1.008 x 4) = 16.04 u and its molar mass is 16.04 g/mol.
Answered by | 25 Jul, 2012, 10:03: AM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:43: PM
CBSE 9 - Chemistry
Asked by jssjj | 19 Jan, 2023, 07:25: PM
CBSE 9 - Chemistry
Asked by mohammedhaqqani.6b | 14 Jun, 2022, 02:51: PM
CBSE 9 - Chemistry
Asked by gauravsingh36428 | 14 Mar, 2022, 07:44: PM
CBSE 9 - Chemistry
Asked by jiyajthakor | 28 Feb, 2022, 07:03: PM
CBSE 9 - Chemistry
Asked by gillsaabjashanpreetsingh3 | 16 Jan, 2022, 01:23: PM
CBSE 9 - Chemistry
Asked by prachisharma772007 | 16 Jan, 2022, 11:12: AM











