ICSE Class 10 Answered
what is meant by lattice energy. How does it affect electrovalent bonding
Asked by kshhitij | 24 Feb, 2013, 12:17: PM
An estimate of the strength of the bonds in an ionic compound can be obtained by measuring the lattice energy of the compound, which is the energy given off when oppositely charged ions in the gas phase come together to form a solid. Higher the lattice energy, the greater will be the case of the forming an ionic compound. The quantity of energy released when free ions combine together to form one mole of a crystal is called lattice energy (U).Lattice energy 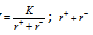 ; is inter nuclear distance.
; is inter nuclear distance.
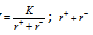 ; is inter nuclear distance.
; is inter nuclear distance.
Answered by | 24 Feb, 2013, 09:16: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by vijayvijay09644 | 06 Mar, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by navedsheikh97658 | 01 Nov, 2023, 04:57: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 18 Jul, 2022, 10:39: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 18 Jul, 2022, 10:33: PM
ICSE 10 - Chemistry
Asked by anubhavsur.140914 | 25 Oct, 2021, 07:31: PM
ICSE 10 - Chemistry
Asked by manasa | 10 Sep, 2021, 06:32: PM
ICSE 10 - Chemistry
Asked by waliaman704 | 29 Jun, 2021, 11:58: AM
ICSE 10 - Chemistry
Asked by manbeersinghahhps | 19 May, 2021, 07:12: PM
ICSE 10 - Chemistry
Asked by aras89009 | 09 May, 2021, 02:23: PM











