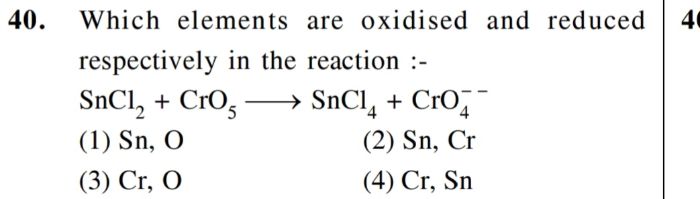CBSE Class 12-science Answered
What is Lanthanoid contraction and what is the factor responsible for that?
Asked by Topperlearning User | 06 Jun, 2016, 01:48: PM
There is an increase in radii from the first (3d) to the second (4d) series of the elements but the radii of the third (5d) series are virtually the same as those of the corresponding members of the second series. This phenomenon is called Lanthanoid contraction. The possible reason for that is the intervention of the 4f orbitals which must be filled before the 5d series of elements begins. The imperfect shielding of one 4f electron by another result in the decrease in the size of the entire 4f n orbitals as the nuclear charge increases along the series.
Answered by | 06 Jun, 2016, 03:48: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 06:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 02:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 10:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 01:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:44: PM