CBSE Class 12-science Answered
what happens when phenol is introduced to litmus while the other alcohols are neutral?
Asked by shaikh sameer | 01 Oct, 2013, 03:31: PM
Alcohols and phenol which contain an -OH group attached to a hydrocarbon are very weak acids. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored.
However, phenol is sufficiently acidic for it to have recognisably acidic properties - even if it is still a very weak acid. A hydrogen ion can break away from the -OH group and transfer to a base.
For example, in solution in water:
![]()
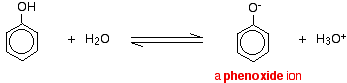
So, it turns blue litmus red.
Answered by | 02 Oct, 2013, 08:56: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by sanyaagrahari8888 | 28 Nov, 2023, 01:07: PM
CBSE 12-science - Chemistry
Asked by bithikapurkait09052005 | 24 Jun, 2022, 08:35: AM
CBSE 12-science - Chemistry
Asked by rohanm | 14 Feb, 2022, 04:49: PM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 28 Nov, 2020, 04:29: PM
CBSE 12-science - Chemistry
Asked by holebasubudihal | 09 Aug, 2020, 02:58: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 12 Jun, 2020, 09:34: PM











