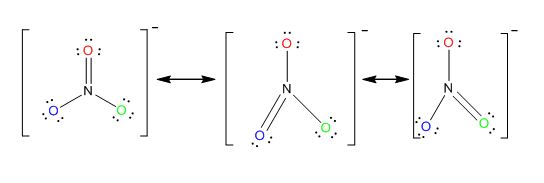
What conditions stabilize a resonance ? Also what are possible resonance structure of nitrate ion ?
Resonance structures are imaginary. They represent extremes of electron location.
In general chemistry, the concept of resonance was introduced through inorganic anions such as NO3-, NO2-, ClO4-, SO42-, CO32-.
The curved arrow shows “movement” of a pair of electrons.
It’s an extremely useful accounting system that lets us keep track of changes in bonding and also in charge.
Since electron pairs are present either in bonds or in lone pairs, there are really only four combinations of “moves”.
The possible resonance structure of nitrate ion:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Classification of Elements and Periodicity in Properties
- 2 Chemical Bonding and Molecular Structure
- 3 States of Matter
- 4 Thermodynamics
- 5 Equilibrium
- 6 Hydrogen
- 7 Hydrocarbons
- 8 Environmental Chemistry
- 9 Solutions
- 10 Chemical Kinetics
- 11 Surface Chemistry
- 12 Biomolecules
- 13 Polymers
- 14 Chemistry in Everyday Life
- 15 Gravitation
- 16 Electromagnetic Waves
- 17 Communication Systems
- 18 Laws of Motion
- 19 Current Electricity
- 20 Cell : The Unit of Life
- 21 Respiration in Plants
- 22 Work, Energy and Power
- 23 Animal Kingdom
- 24 Atomic Structure
- 25 Kinematics
- 26 Evolution
- 27 Ecosystem
- 28 Chemical Thermodynamics
- 29 Redox Reactions and Electrochemistry
- 30 p-Block Elements
- 31 d - and f - Block Elements
- 32 Some Basic Principles of Organic Chemistry
- 33 Organic Compounds Containing Halogens
- 34 Organic Compounds Containing Oxygen
- 35 Organic Compounds Containing Nitrogen
- 36 Physics and Measurement
- 37 Rotational Motion
- 38 Properties of Solids and Liquids
- 39 Kinetic Theory of Gases
- 40 Oscillations and Waves
- 41 Electrostatics
- 42 Magnetic Effects of Current and Magnetism
- 43 Electromagnetic Induction and Alternating Currents
- 44 Optics
- 45 Dual Nature of Matter and Radiation
- 46 Atoms and Nuclei
- 47 Electronic Devices
- 48 The Living World
- 49 Co-ordination Compounds
- 50 Biological Classification
- 51 Reproduction in Organisms
- 52 Sexual Reproduction in Flowering Plants
- 53 Human Reproduction
- 54 Reproductive Health
- 55 Principles of Inheritance and Variation
- 56 Molecular Basis of Inheritance
- 57 Biotechnology : Principles and Processes
- 58 Biotechnology and its Applications
- 59 Human Health and Disease
- 60 Microbes in Human Welfare
- 61 Strategies for Enhancement in Food Production
- 62 Organisms and Populations
- 63 Biodiversity and Conservation
- 64 Environmental Issues
- 65 Plant Kingdom
- 66 Morphology of Flowering Plants
- 67 Anatomy of Flowering Plants
- 68 Structural Organisation in Animals
- 69 Cell Cycle and Cell Division
- 70 Mineral Nutrition
- 71 Photosynthesis in Higher Plants
- 72 Plant Growth and Development
- 73 Digestion and Absorption
- 74 Breathing and Exchange of Gases
- 75 Body Fluids and Circulation
- 76 Excretory Products and their Elimination
- 77 Locomotion and Movement
- 78 Neural Control and Coordination
- 79 Chemical Coordination and Integration
- 80 Transport in Plants
- 81 Purification and Characterisation of Organic Compounds
- 82 s-Block Element (Alkali and Alkaline Earth Metals)
- 83 Solid State
- 84 Some Basic Concepts in Chemistry
- 85 General Principles and Processes of Isolation of Metals
- 86 Principles Related to Practical Chemistry