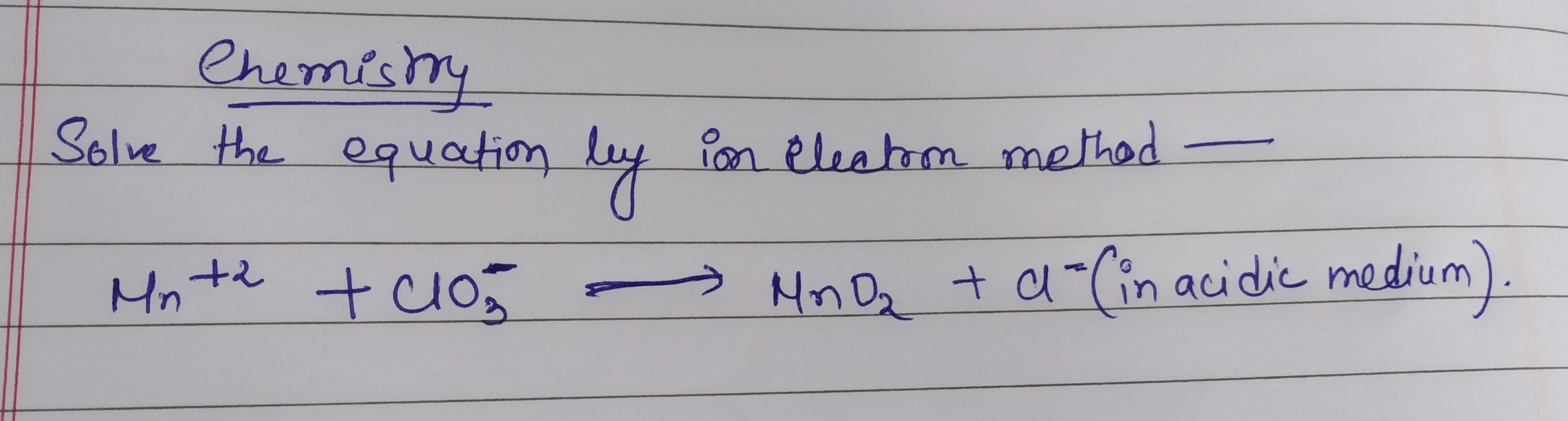
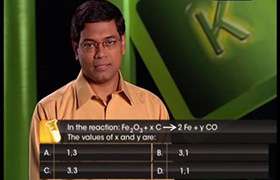CBSE Class 11-science Answered
Step - I Write the skeletal equation and indicate the Oxidation number of all the elements which appear in the skeletal equation above their respective symbols.
Step – II Find out the species which are oxidized and which are reduced.
Step – III Split the skeletal equation into two half reactions, i.e. oxidation half reaction and reduction half equation.
Step – IV Balance the two half equations separately by the rules described below:
(a) In each half reaction, first balance the atoms of the elements which have undergone a change in oxidation number.
(b) Add electrons to whatever side is necessary to make up the difference in oxidation number in each half reaction.
(c) Balance charge by adding H+ ions if the reaction occurs in the acidic medium and by adding OH- ions if the reaction occurs in basic medium.
(d) Balance oxygen atoms by adding required number of H2O molecules to the side deficient in O atoms.
(e) In the acidic medium, H atoms are balanced by adding H+ ions to the side deficient in H atoms. However, in the basic medium H atoms are balanced by adding H2O molecules equal in number to the deficiency of H atoms and equal OH- ions are included in the opposite side of the equation. Remove the duplication, if any.
Step – V The two half reactions are then multiplied by suitable integers so that the total number of electrons gained in one half reaction is equal to the number of electrons lost in the other half reaction. The two half reactions are then added up.