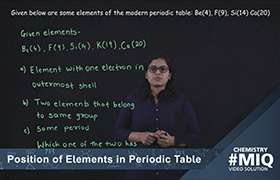
CBSE Class 10 Answered
Two elements with symbol X (atomic no. 11) and Y (atomic no. 13) are
placed in the III period of the modern periodic table -
(i) Which amongst the two has more metallic character?
(ii) Calculate the valency of each element.
(iii) Element ‘Y’ is smaller than ‘X’ in terms of atomic size. Is the
statement true, justify?
Asked by Kunwar Digvijay Singh Tanwar | 02 Mar, 2013, 05:51: PM
i) X has a more metallic character as metallic character decreases as we go from left to right in a period.
ii) Valency of X= +1
Valency of Y= +3
iii) The statement is true. As we move from left to right along a period the atomic size decreases. This is because of higher number of protons in the nucleus which leads to a higher force of attraction between the nucleus and electrons,
Answered by | 02 Mar, 2013, 07:47: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM