CBSE Class 12-science Answered
The well known mineral fluorite is chemically
calcium fluoride. It is know that in one unit cell of this mineral, there are
4 Ca2+ ions and 8 F- ions and that Ca2+ ions
are arranged in fcc lattice. The F- ions fill all the tetrahedral
holes in the face centred cubic lattice of Ca2+ions. The edge of
the unit cell is 5.46 10-8 cm in length. The density of the
solid is 3.18 g cm-3. Use this information to calculate Avogadro's
number (Molar mass of CaF2 = 78.08 g mol-1)
10-8 cm in length. The density of the
solid is 3.18 g cm-3. Use this information to calculate Avogadro's
number (Molar mass of CaF2 = 78.08 g mol-1)
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
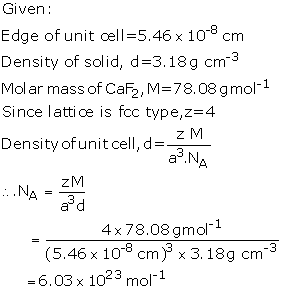
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 07:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 09:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




