CBSE Class 12-science Answered
The vapor pressure of water is 12300 Pa at 27 C. Calculate the vapor pressure of 0.5 M solution of a non dissociating/non associating solute in it.
Asked by lekhakarthikeyan | 28 Aug, 2018, 06:29: AM
Given:
Vapour pressure of H2O p01 =12300 kPa
=12.3 Pa
Molality of solution =0.5 m
Vapour pressure of solution =p1
1 molal solution means 1 mole of solute in 1000 g of solvent
0.5 molal solution means 0.5 mole of solute in 1000 g of solvent
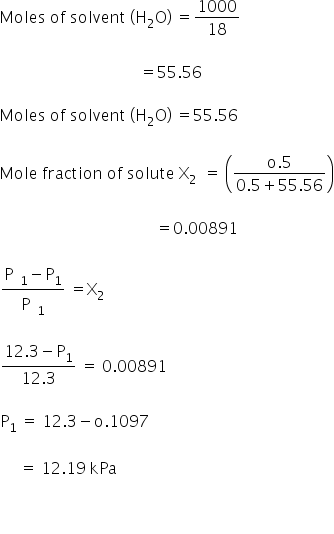
Vapour pressure of solution is 12.19 kPa
Answered by Varsha | 28 Aug, 2018, 02:18: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by premkhare2006 | 24 Jan, 2024, 09:50: AM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 01:25: PM
CBSE 12-science - Chemistry
Asked by kaushikmisty07 | 31 Dec, 2023, 11:42: AM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM








