CBSE Class 11-science Answered
The values of heatformation of SO2 and SO3 are-398.2 KJ and 198.2 KJ.The heat of formation of the reaction
SO2+1/2 O2 gives SO3 will be
1.-200 KJ
2.356.2 KJ
3.-200 KJ
4.-396.2 KJ
Asked by TVISHA Bhatt | 29 Aug, 2014, 11:13: AM
Heat of formation of SO2 is 398.2 kJ.
S + O2 → SO2
Reverse the above reaction,
SO2 → S + O2 ……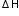 = -398.2 kJ ...... (i)
= -398.2 kJ ...... (i)
Heat of formation of SO3 is 198.2 kJ
S + 3/2 O2 → SO3 ......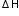 = 198.2 kJ .....(ii)
= 198.2 kJ .....(ii)
SO2 + 1/2 O2 → SO3 ......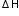 = -398.2 + 198.2 = -200 kJ
= -398.2 + 198.2 = -200 kJ
Answered by Arvind Diwale | 31 Aug, 2014, 09:14: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM