CBSE Class 11-science Answered
The salts of alkali metals are the most ionic salts known. Although lithium is an alkali yet its compounds, particularly halides, are slightly covalent in nature.
Asked by Topperlearning User | 28 Jun, 2016, 01:54: PM
Li+ ion has small size and has maximum tendency to distort the electron cloud of the anion by withdrawing the electrons from the negative ion towards itself. This tendency to distort the electron cloud of the negative ion by the positive ion is known as polarization. It causes the charges on the ions to become less since some of its charges get neutralized. This leads to the covalent character of the bond.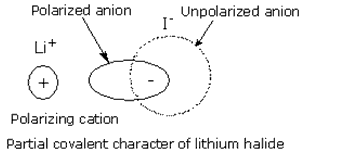
Larger the polarization, larger will be the covalent character of the bond. Thus, Li+ being small in size polarizes the anion and results in decrease of the positive charge on Li+ ion. Therefore, lithium halides are covalent in nature.
Answered by | 28 Jun, 2016, 03:54: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:49: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 03:32: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 03:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 03:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 01:54: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM





