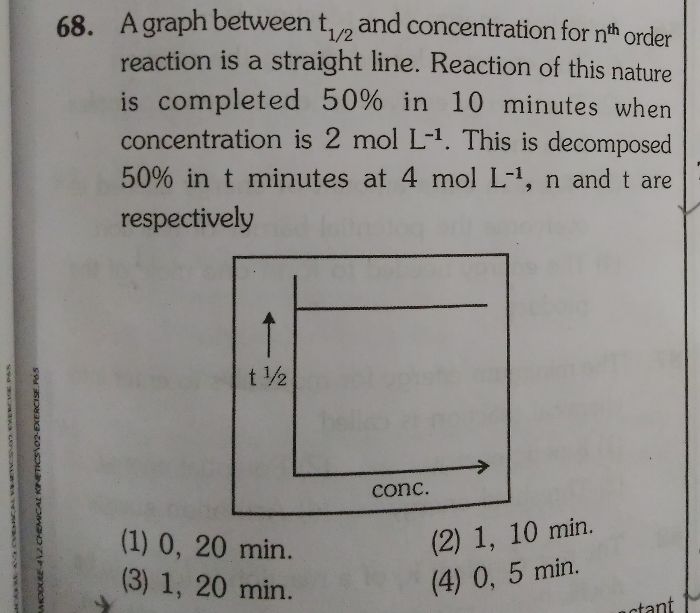
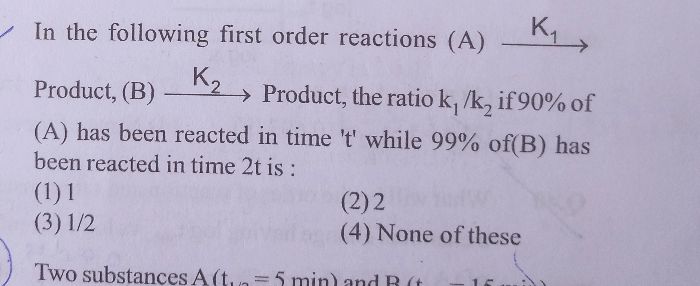CBSE Class 12-science Answered
the rate constant for first order decomposition of H2O2 os given by the following equation
Log k=14.34-1.25*10 to the power 4 k/T calculate Ea for given reaction and at what temperature will its half period be 256 minutes?
please solve it by arrhineus equation
Asked by Shahwar | 06 Jul, 2015, 12:56: PM
Answered by Prachi Sawant | 07 Jul, 2015, 10:40: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by cjam41665 | 10 Oct, 2021, 12:56: AM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM