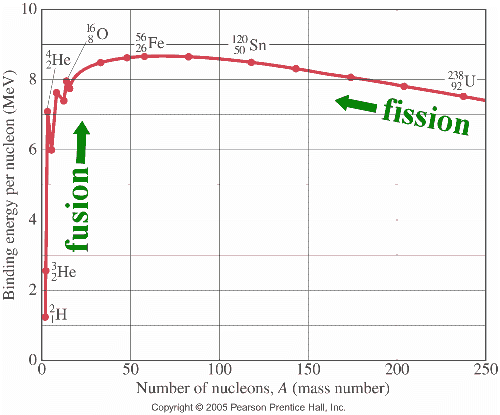
The above graph shows the Binding energy per nucleon as a function of Number of nucleons. It can be seen that for lower mass number some peaks are observed that indicates for specific mass number the binding energies are higher compare to the neighbouring mass numbers. In addition to this peculiar behaviour nuclear physicists observed the discrepancy betwen the predicted binding energy and measured values for some nuclei.
Nuclear physicists developed a simplest nuclear structure model that is known as liquid drop (LD) model to predict the binding energy of nuclei. In this model nuclei are assumed to behave in a similar way like molecuels in liquid. Just like molecues are held together by short range VanderWalls force that is only between near neighbours, it is assumed, nucleons are held by short range nuclear forces that appears to act only between neighbouring nucleus. But the liquid drop model could not explain the peaks shown in the above graph and the discrepancy betwen the predicted binding energy and measured values for some nuclei
Hence nuclear physicists developed the nuclear shell model. The bindng energies predicted by LD model under estimate the actual binding energies of 'magic nuclei' for which either the number of neutrons N = (A-Z) or number of protons Z is equal to one of the following "magic numbers"
2,8,20,28..............etc.
This is particularly case for "doubly magic" nuclei in which both the numbers of neutrons and number of protons are equal to magic number. These magic numbers can be explained in terms of shell model of the nucleus, which considers each nucleon to be moving in some potential due to the presence of other nucleus. The shell model classifies the energy levels in terms of quantum numbers n.l.j in the same way as the energy levels of individual electrons are clasified in atomic physics.
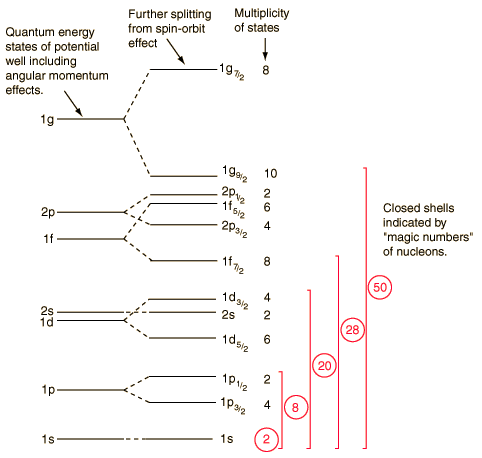
From the theoretical development and calculations, it turns out that for such a potential assumed by shell model, the lowest energy level is 1s as shown by the above figure. This 1s level can contain upto 2 protons or neutrons. Then comes 1p which contains upto a further 6 protons( or neutrons). this explains the first 2 magic numbers 2 and 8. Then there is the level 1d, but this is quite close in energy to 2s so that they form the same shell. this allows a further 2+10 protons(or neutrons) giving us the next magic number 20. Like this the shell model explains other magic numbers.
If the magic numbers are explained by shell model, it can be concluded nuclear energy levels are not continuous, there are only allowed shells of energy levels and these shells are occupied by specific number of nucleons. When the number of nucleons of the nuclei is turn out to be the accumulated numbers of fully completed closed shell, then the element is highly stable and the binding energy graph shows the peak for these particular nuclei.
For further details, students are advised to refer the following references.
(1) http://www.personal.soton.ac.uk/ab1u06/teaching/phys3002/course/05_shell.pdf
(2) http://hyperphysics.phy-astr.gsu.edu/hbase/Nuclear/shell.html