CBSE Class 9 Answered
The latent heat of vaporisation of water at 100degree Celsius is 540,find entropy increase when one mole of water at 100deg.cel is evaporate.
Asked by amitjena226 | 04 Feb, 2018, 10:30: AM
Formula:
dG = dH - TdS
At 1000C water is boiling hence dG = 0
therefore,
TdS = dH
dS= dH / T
Given :
latent heat of vaporisation dH = 540
T = 1000C = 373 K
we get,
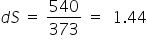
Answered by Ramandeep | 04 Feb, 2018, 06:46: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rehanpinjari94.9dgatl | 11 Oct, 2021, 10:07: PM
CBSE 9 - Chemistry
Asked by shivjaiswal30.9dgatl | 02 Oct, 2021, 10:07: PM
CBSE 9 - Chemistry
Asked by dambalshoba | 06 Sep, 2020, 09:08: PM
CBSE 9 - Chemistry
Asked by harshilmodi74.tl | 16 Apr, 2020, 11:16: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 08 May, 2014, 09:31: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 08 May, 2014, 09:31: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 08 May, 2014, 09:32: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 08 May, 2014, 09:33: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 10 Jul, 2014, 10:48: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 08 May, 2014, 09:34: AM