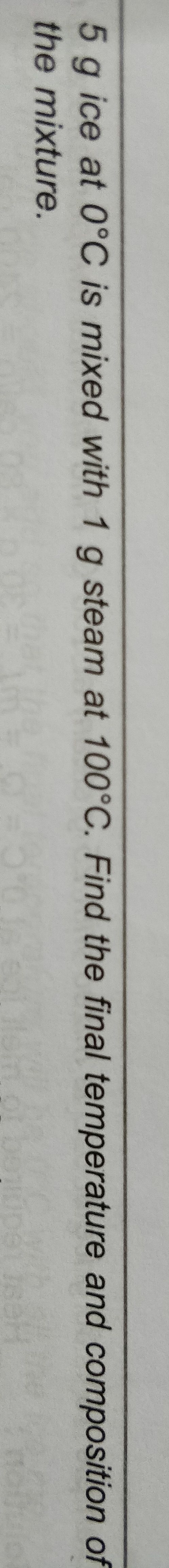NEET Class neet Answered
The equation of a certain gas can be written as ( t^7/5)/( p^2/5 )= const. Its specific heat at constant volume will be
Asked by shruthikamath | 29 Mar, 2019, 08:13: AM
The given equation P^2/5×T^7/5 = constant
Rearranging we get P^2×T^7 = constant
Replacing the value of T from PV =net
We get
P×V^7/9= constant
For any polytropic process PV^z = constant heat capacity is given as
Cv = R/(γ-1)
Where y is the adiabatic constant and for any case its value must be greater than 1.
For the given data it's coming under polytropic process.
Answered by Ankit K | 29 Mar, 2019, 10:21: PM
NEET neet - Physics
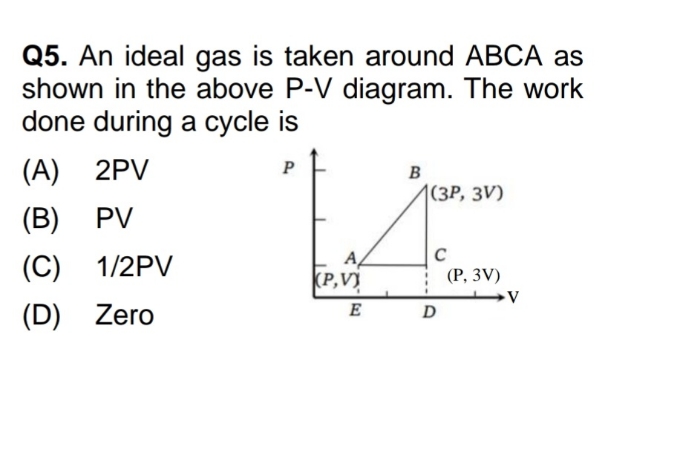
Asked by yadavaradhana9335 | 19 Feb, 2024, 04:53: PM
NEET neet - Physics
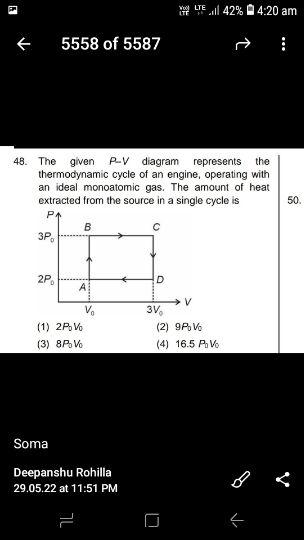
Asked by sujitjana971 | 18 Dec, 2022, 05:23: PM
NEET neet - Physics
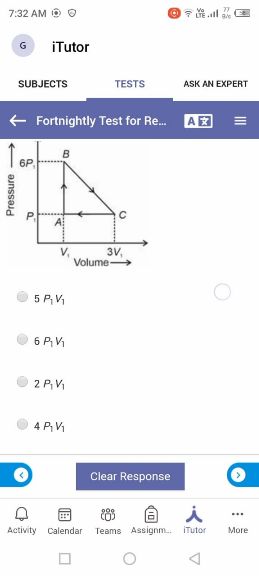
Asked by takshitashu46 | 09 Feb, 2022, 06:01: PM
NEET neet - Physics
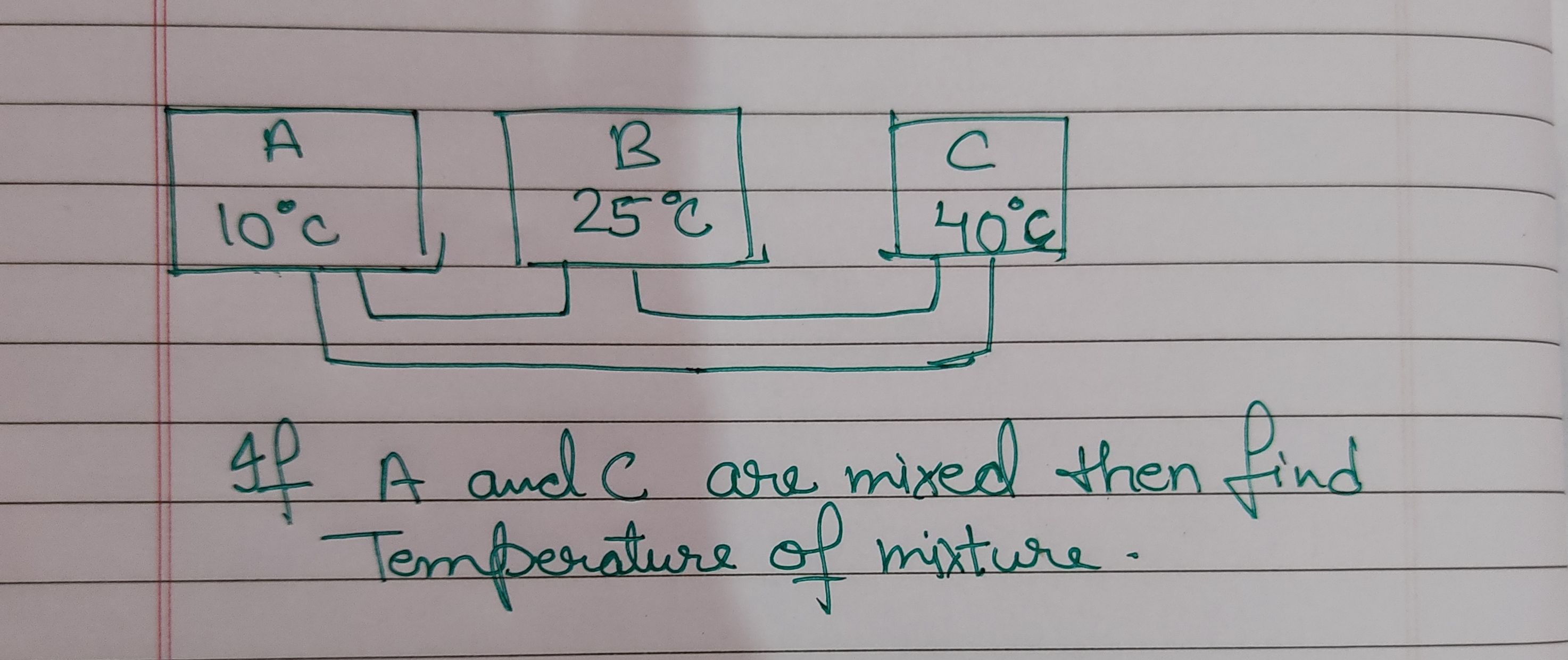
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 05:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 05:23: PM