CBSE Class 12-science Answered
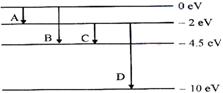
The energy levels of
a hypothetical atom are shows below. Which of the shown transitions will
result in the emission of a photon of wavelength 275 nm?
Which of these
transitions correspond to emission of radiation of (i) maximum and (ii)
minimum wavelength?

Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Wavelength
associated : 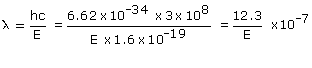
For A:
Energy change: E1-E2 = 0 - (-2) = 2 eV
So wavelength of the radiation emitted is:
![]()
![]()
(wavelength emitted is 256 nm, i.e, radiation B)
![]()
![]()
Maximum wavelength : emission A
Minimum wavelength: emission D
Answered by | 04 Jun, 2014, 03:23: PM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 09:26: PM
CBSE 12-science - Physics
Asked by Akshatkuma2004 | 04 Jul, 2021, 04:04: PM
CBSE 12-science - Physics
Asked by rsrakesh932 | 12 Jun, 2020, 04:38: PM
CBSE 12-science - Physics
Asked by yadavnitish688 | 14 Dec, 2019, 06:04: AM
CBSE 12-science - Physics
Asked by sidiz.shrestha07 | 26 May, 2019, 06:42: AM
CBSE 12-science - Physics
Asked by pardeepkumar2281 | 28 Feb, 2019, 07:24: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 03:54: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 03:51: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:02: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:01: PM

