CBSE Class 12-science Answered
The densities of the various forms of carbon are shown in the table below. Explain the drastic change in density between the various forms of carbon.
Form of Carbon
Density
(grams/cubic centimeter)
Amorphous carbon
1.8 - 2.1
Buckminsterfullerene C60
1.69
Graphite
1.9 - 2.3
Diamond
3.50 - 3.53
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
The density is a property that measures how efficiently a substance is spatially packed. The relationship between the mass, volume, and density of a substance is:
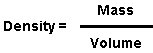
This drastic change in density between the various forms of carbon is a consequence of the crystalline structure that each substance adopted. The macroscopically measured density is usually slightly different than that measured using X-ray diffraction because of defects and/or impurities within the sample.
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 07:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 09:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




