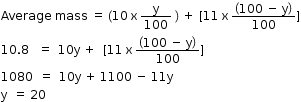CBSE Class 9 Answered
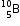 .
(i) What does the figures 5 and 10 indicate?
(ii) How many number of electrons does this element contain?
(iii) The relative atomic mass of Boron is 10.8 u. Calculate the percentage of its isotopes
.
(i) What does the figures 5 and 10 indicate?
(ii) How many number of electrons does this element contain?
(iii) The relative atomic mass of Boron is 10.8 u. Calculate the percentage of its isotopes 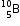 and
and 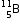 , occurring in nature.
, occurring in nature.(a) Chlorine atom (Cl) has atomic number 17. It contains 17 protons and 17 electrons.
Chlorine ion (Cl-) is formed when Cl gains one electron.
So, Cl- has 18 electrons and 17 protons.
Therefore, the electronic configuration of Cl- = 2, 8, 8.
(b) Atomic number of Cl- = Number of protons = 17
Mass number of Cl- will be the same as Cl i.e. 35.
(c) Valency is defined as the combining capacity of an atom.
For a non-metallic element, it is equal to eight minus the number of electrons present in outermost shell.
Here, Cl- has 8 electrons in the outermost shell, therefore valency of Cl- = 8 - 8 = 0
OR
(a) (i) 5 is the atomic number.
10 is the atomic mass number.
(ii) Number of neutrons = Mass number - Atomic number
= 10 - 5
= 5
(b) Let the percentage of 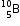 be y in the sample.
be y in the sample.
Let the percentage of 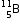 will be (100 - y)
will be (100 - y)