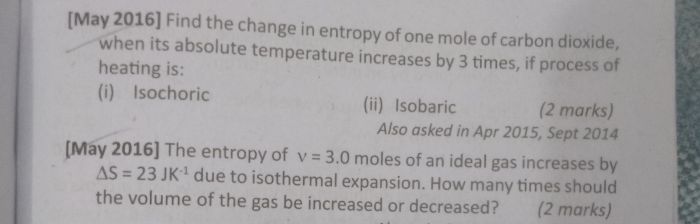JEE Class main Answered
steam at 100 degree celsius is passed into 1 kg of water contained in a calorimeter at 9 degree celsius till the temperature of water and calorimeter is increased to 90 degree celsius. The mass of steam condensed is nearly
( water equivalent of calorimeter =0.1 kg
specific heat of water =1calg-1degree celsius inverse
and latent heat of vaporisation =540calg-1
Asked by Charishmachowdari0909 | 30 Mar, 2019, 09:42: AM
Let x be the mass of steam condensed. The heat obtained calorimeter with water is equal the heat from condensing x kg of steam and the heat from cooling x kg of water to the final temperature of calorimeter with water. x∙L + x∙c∙(T3 − T2 ) = (m + mc)∙c∙(T2 − T1 ), where L = 540 cal/g – latent heat of steam, c = 1 cal/g∙degree – specific heat of water,Read more on Sarthaks.com - https://www.sarthaks.com/106929/steam-at-100c-is-passed-into-1-1-kg-of-water-contained-in-a-calorimeter
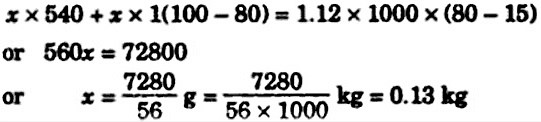
Answered by Ankit K | 31 Mar, 2019, 08:17: PM
JEE main - Physics
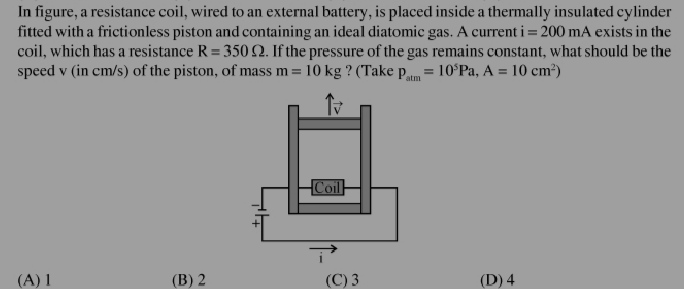
Asked by hridayjayaram085 | 12 Jan, 2024, 05:50: PM
JEE main - Physics
Asked by ashainy91829 | 06 Nov, 2023, 01:08: PM
JEE main - Physics
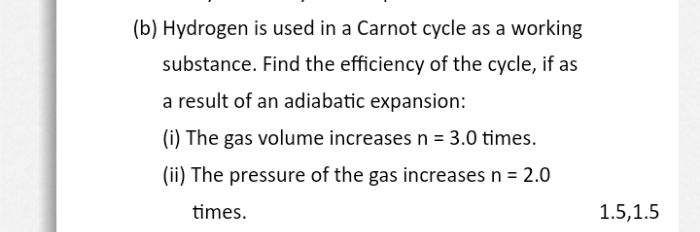
Asked by ghrushi3 | 02 Nov, 2023, 10:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 10:03: PM
JEE main - Physics
Asked by manvirsingh2242 | 21 Jun, 2022, 04:35: PM
JEE main - Physics
Asked by manvirsingh2242 | 20 Jun, 2022, 12:32: PM
JEE main - Physics
Asked by manvirsingh2242 | 18 Jun, 2022, 06:27: PM
JEE main - Physics
Asked by manvirsingh2242 | 11 Jun, 2022, 09:16: AM